Utilization of Wolinella succinogenes asparaginase to treat diseases associated with asparagine dependence
a technology of asparagine dependence and wolinella succinogenes, which is applied in the direction of drug compositions, peptide/protein ingredients, immunological disorders, etc., can solve the problems of life-threatening coagulopathy, pronounced immunosuppression, and wide range of host, and achieves potent anti-neoplastic activity and high efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Animals and Cell Lines
[0089] The murine model animals utilized in these experiments were Balb / C or C3H mice of 9 to 12 weeks in age (Jackson Laboratories, Bar Harbor, Me.).
[0090] The therapeutic activity of L-asparaginases was determined utilizing the 6C3HED Gardner's lymphosarcoma (Gardner, W. U., Cancer Res., vol. 4: 73 (1944)) and P1798 lymphosarcoma cell lines (ATCC) which as ascites tumors in C3H and Balb / cc mice, respectively. Alternately, the two lymphosarcoma cell lines were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum. The 6C3HED Gardner's lymphosarcoma originated in the thymus of C3H mice which were initially given high doses of estradiol. The lymphosarcoma was subsequently perpetuated by serial transplantation in the C3H mice.
example 3
Isolation of W. succinogenes Genomic DNA
[0091] Genomic DNA from W. succinogenes was extracted from bacteria grown in basal medium. Typically, bacterial cells from a 50 ml of culture were collected by centrifugation and resuspended by gentle vortexing in 1.5 ml TE buffer (pH 7.0). To the cell suspension was added 15 .mu.l of 10% SDS to give a final concentration of 0.1% and 3 .mu.l of a 20 mg / ml stock solution of proteinase K. The mixture was then incubated at 37.degree. C. for approximately 60 minutes, followed by several phenol / chloroform extractions. The genomic DNA was ethanol precipitated and collected by centrifugation. The W. succinogenes genomic DNA so isolated was sufficiently pure to use in high stringency PCR amplification.
example 4
PCR Amplification of W. succinogenes Asparaginase Sequences
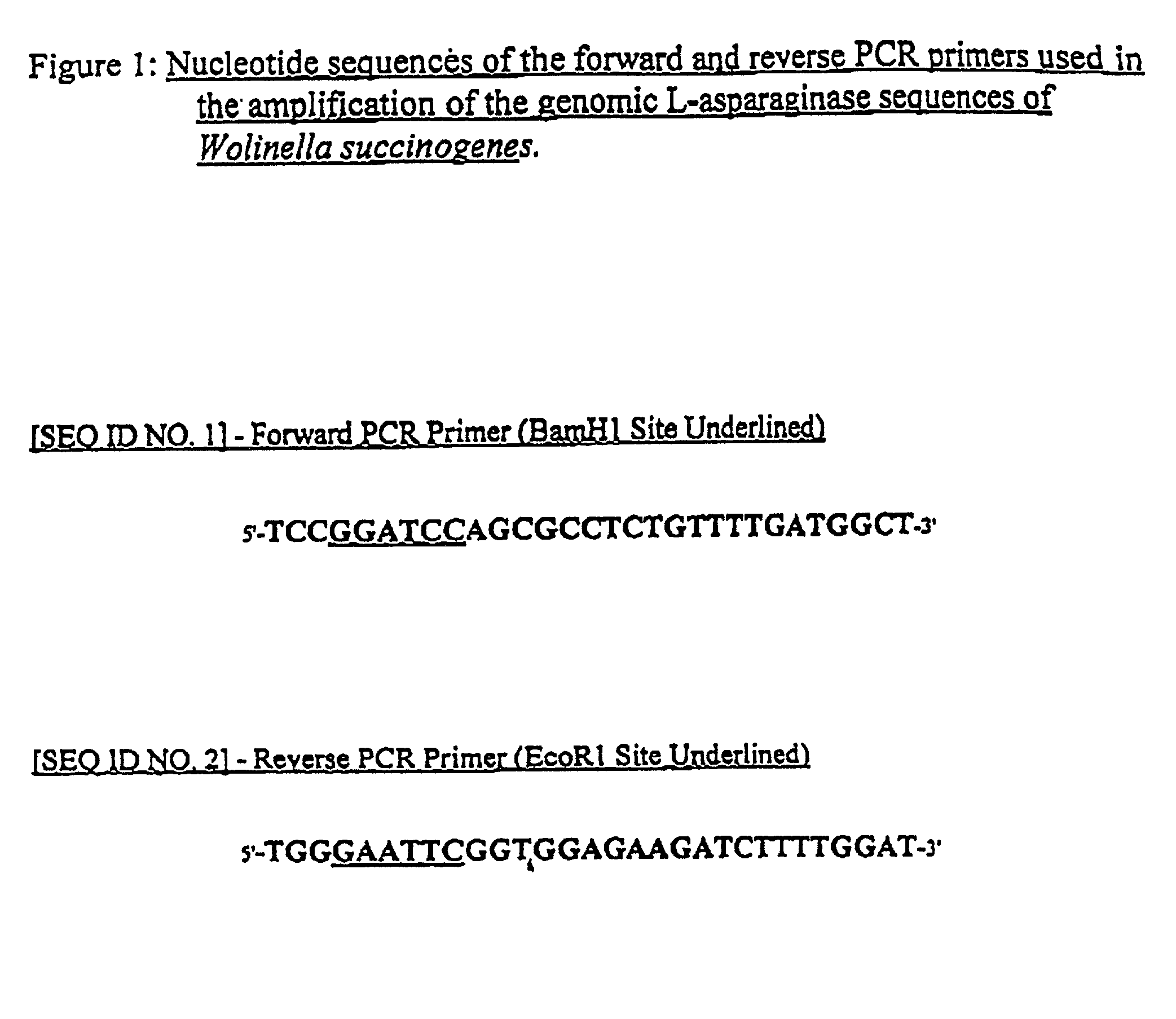
[0092] The nucleotide sequence of a 2.5 kb Hind III fragment containing the 993 nucleotide coding region of W. succinogenes asparaginase was published in 1995. See GenBank accession number X89215. The elucidation of this sequence facilitated the synthesis of primers specific for PCR amplification of the gene coding, for the W. succinogenes enzyme. As illustrated in FIG. 1, the forward and reverse W. succinogenes asparaginase-specific PCR primers forward and reverse had the following sequences:
1 5'-TCCGGATCCAGCGCCTCTGTTTTGATGGCT-3' Forward PCR Primer [SEQ ID NO. 1] (BamHI] Restriction Site Underlined) 5'-TGGGAATTCGGTGGAGAAGATCTTTTGGAT-3' Reverse PCR Primer [SEQ ID NO. 2] (EcoR1 Site Restriction Underlined)
[0093] It should be noted that the genomic W. succinogenes asparaginase coding sequence does not naturally contain either a BamH1 or EcoR1 restriction site. However, PCR amplification utilizing these aforementioned primers i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| optical density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com