Aminoketone asymmetric reductase and nucleic acid thereof
An amino acid and amino ketone technology, applied in the directions of oxidoreductase, genetic engineering, plant genetic improvement, etc., can solve the problems of unfavorable economy, low yield, and many processes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0222] (refinement of aminoketone asymmetric reductase)
[0223] Into a test tube containing 5 ml of medium with a composition (pH 7.0) of 1% glucose, 0.3% dipotassium hydrogen phosphate, 0.02% magnesium sulfate heptahydrate, 1.5% peptone, 0.2% sodium chloride, and 0.1% yeast extract Rhodococcus erythropolis MAK-34 was implanted, and shaking culture was carried out at 28°C for 3 days, and the obtained test sample was inoculated into 500 ml medium having the above composition, and shaking culture was carried out at 28°C for 3 days. This pre-culture solution was inoculated into a 501 medium having the above-mentioned composition supplemented with 0.1% of 1-amino-2-hydroxypropane, and cultured at 28° C. for 2 days.
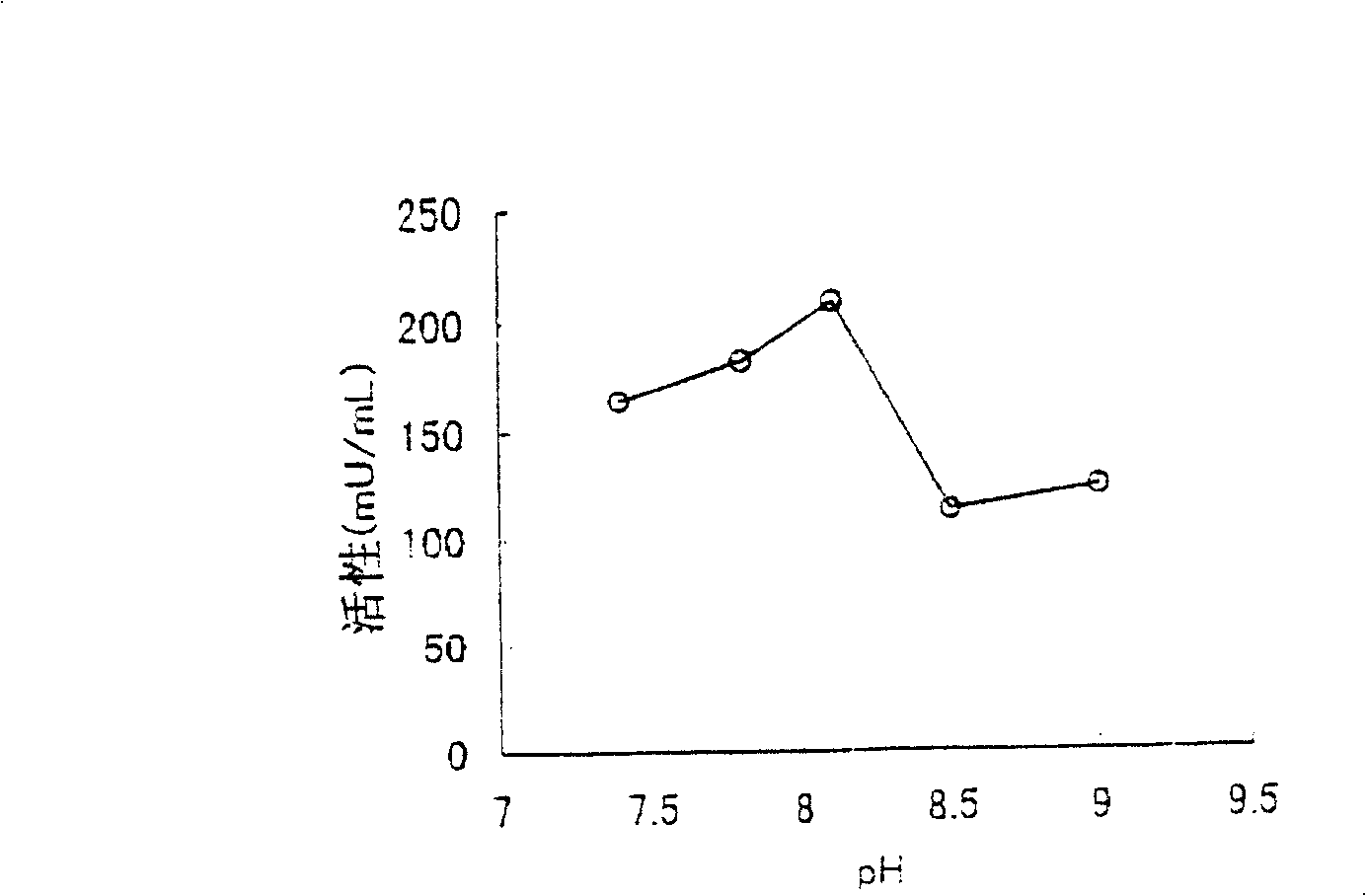
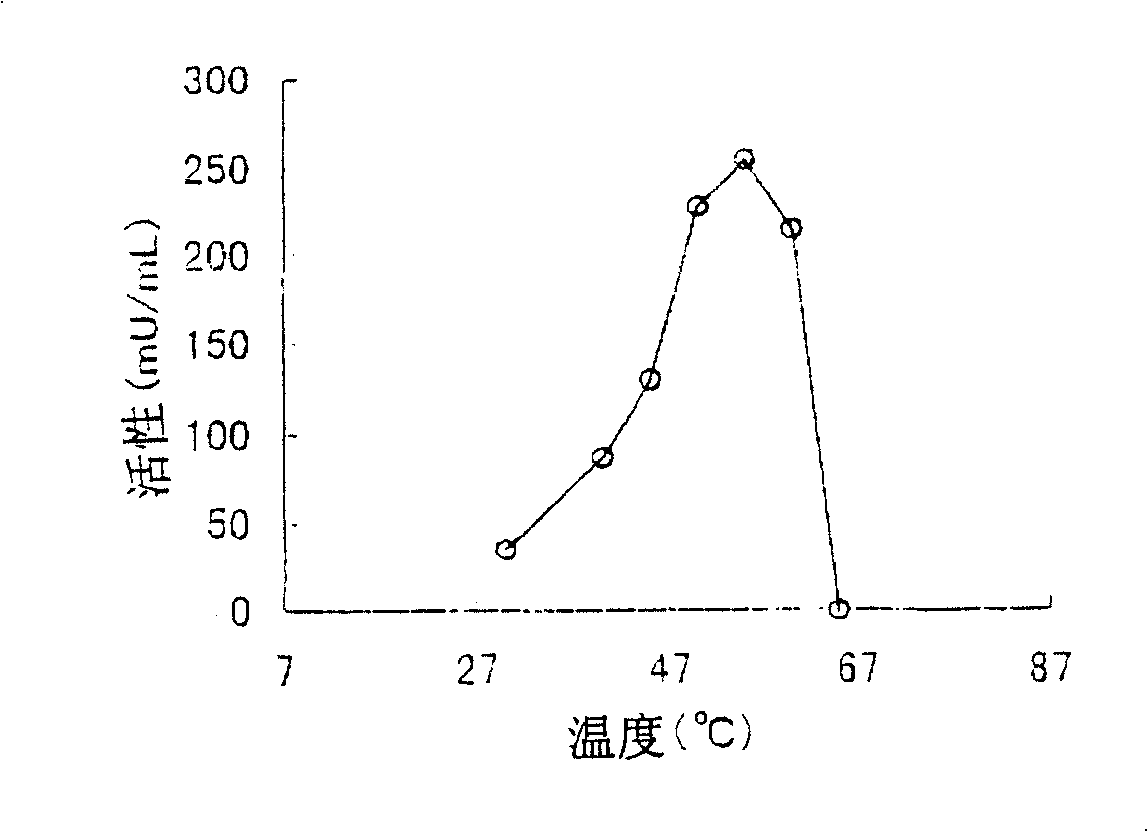
[0224] The above-mentioned culture solution was processed with a centrifuge to obtain 3785 g of wet bacteria. In 1 kg of the thalli, add 1000 ml (pH 8.5) of 10 mM tris hydrochloric acid buffer solution containing 10% glycerin, 1 mM nickel chloride, 1 mM nicotinic ac...
Embodiment 2
[0238] (Cloning of Aminoketone Asymmetric Reductase Gene and Discovery in Escherichia coli)
[0239] (1) Determination of the amino acid partial sequence of the refined enzyme
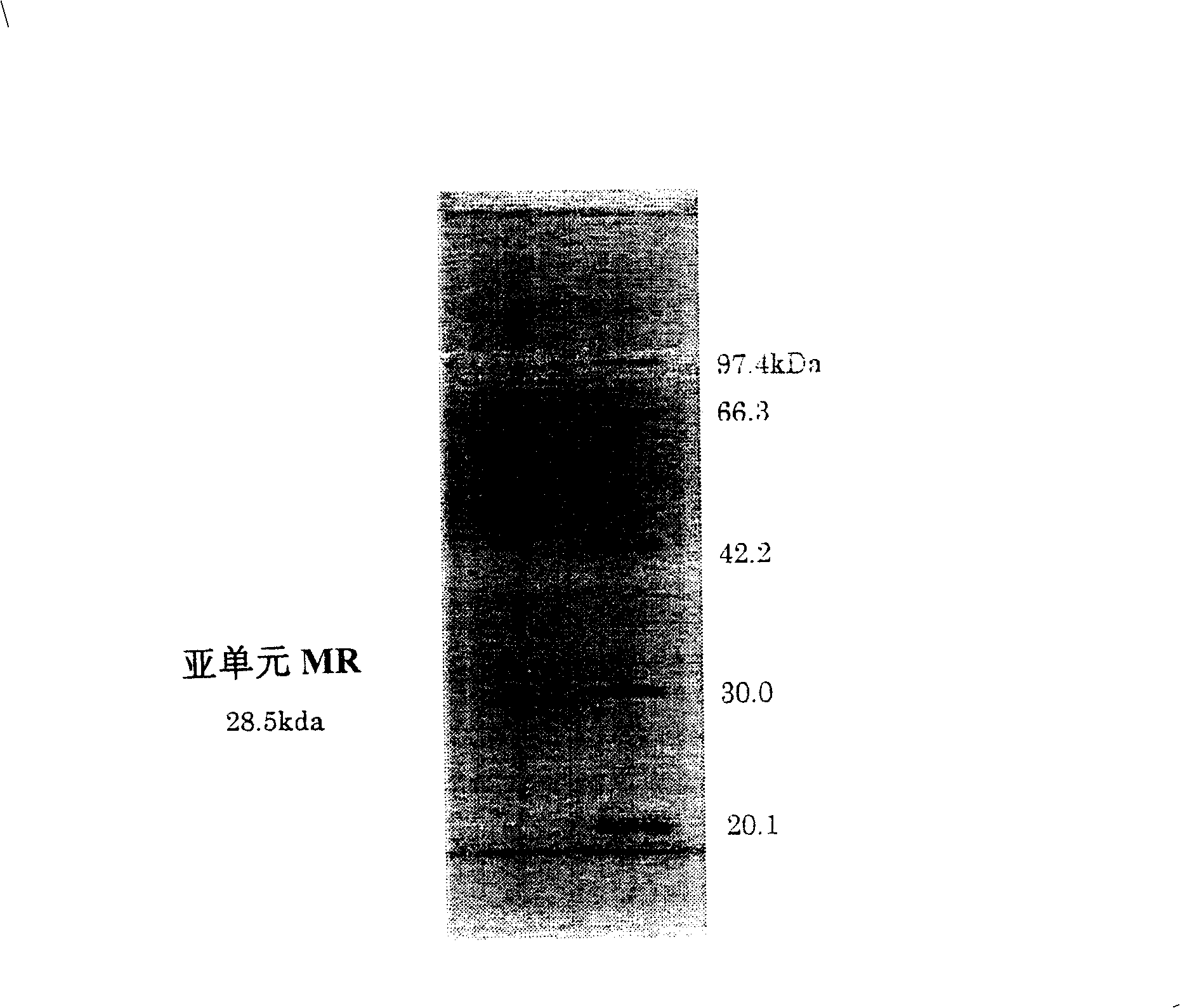
[0240] Dissolve 1 nmol of lyophilized aminoketone asymmetric reductase (subunit molecular weight about 28,500) in 50 μl of 50 mM Tris hydrochloride buffer (pH 8.6) containing 8 M urea, and heat at 37 ° C 1 hour to get modified. Then 50 μl of 50 mM tris hydrochloride buffer solution (pH 8.6) was added to make the urea concentration 4M. Add 0.5 μl (0.006 nmol, E / S=1 / 167) of lysyl endopeptidase (Wako Pure Chemical Industries, Ltd.), and digest at 30° C. for 6 hours. The obtained digested peptides were fractionated by a reverse phase column (Amersham Biosciences), and the amino acid sequence was analyzed with a 476A protein sequencer from ABI Company. The result is as follows.
[0241] ①MFNSIEGRSVVVTGGSK (N-terminal) (sequence number 3)
[0242] ②RLGEMTSEDMDSVFGVNVK (serial number 4)
[0243] ③AAQMGF...
Embodiment 3
[0329] (Formation of d-(1S,2S)-pseudoephedrine)
[0330] Each of the microorganisms shown in Table 1 was implanted in 5 ml of medium containing 1% glucose, 0.5% peptone, and 0.3% yeast extract, and cultured with shaking at 30° C. for 48 hours. After centrifuging the culture solution to obtain bacterial cells, the bacterial cells were placed in a test tube, and 1 ml of a 0.1 M sodium phosphate buffer solution (pH 7.0) was added thereto to suspend them. Furthermore, 1 mg of d 1-2-methylamino-propiophenone hydrochloride was added to the suspension, and the mixture was shaken at 30° C. for 24 hours to perform a reaction. After the reaction was finished, the reaction solution was centrifuged to remove the thalline, and the supernatant was added to HPLC to obtain optically active pseudoephedrine (made by μBondapakphenyl Waters, 4mm in diameter and 300mm long, and the eluent was 0.05M sodium phosphate buffer solution ( Contains acetonitrile 7%), pH 5.0, flow rate 0.8ml / min, detectio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com