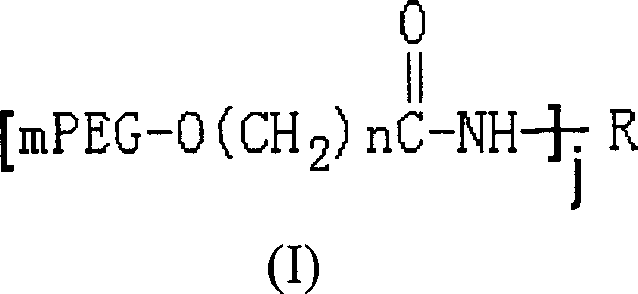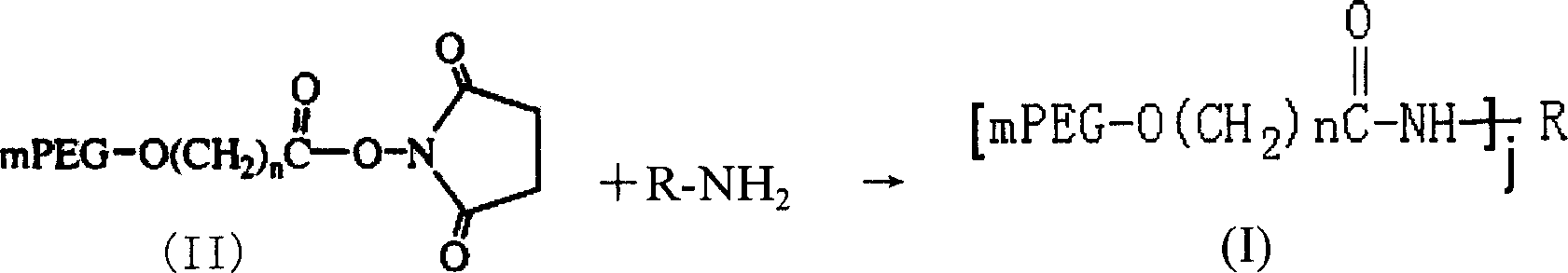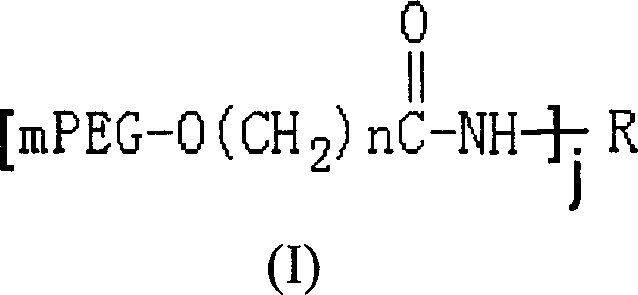Polyethylene glycol modified L-asparaginyl amine enzyme
A technology of asparaginase and polyethylene glycol, which is applied in the directions of hydrolase, medical preparations containing active ingredients, peptide/protein components, etc., can solve the problem of increasing the immunogenicity of aspart, the instability of modified products, and easy hydrolysis. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Selection of Modification Conditions for L-Asparaginase by Methylbutyrate Methoxypolyethylene Glycol Succinimidyl Ester 5000 (mPEG-SMB-5000)
[0092] Selection of reaction pH value: Take 0.5ml of 1mg / ml L-asparaginase solution each, put them in 6 test tubes with stoppers, add 1ml of buffer solution with different pH values respectively to make the pH value of the solution 4.5, 5.5, 6.5, 7.5, 8.5, 9.5, then add 1.773 mg of mPEG-SMB-5000 solid, dissolve, mix well, and react at 25°C for 30 minutes to terminate the reaction. Compare the modification rate and determine the modification condition.
[0093] The results showed that the polyethylene glycol-modified L-asparaginase could be obtained under these conditions, and the modification rate was the highest when the pH value was 9.5.
[0094] Selection of reaction temperature: Take 2ml of 2mg / ml L-asparaginase solution, add 2ml of borax-sodium hydroxide buffer to make the pH of the solution 9.5, then add 14.2mg of mPEG-S...
Embodiment 2
[0101] Preparation of L-asparaginase modified by methylbutyrate methoxypolyethylene glycol succinimidyl ester 5000 (mPEG-SMB-5000)
[0102] Modification reaction:
[0103] Take 2ml of 2mg / ml L-asparaginase solution, add borax-sodium hydroxide buffer to make the pH of the solution 9.5, then add 7.092mg of mPEG-SMB-5000 solid, dissolve, mix well, and react at 25°C After 30 min, 100 mg of glycine was added to terminate the reaction.
[0104] Ion exchange chromatography:
[0105] Take the above reaction solution, with 0.05M Na 2 HPO 4 -NaH 2 PO 4 , pH8.0 buffer solution was ultrafiltered three times, fully balanced, and separated on the column. The chromatographic conditions are as follows:
[0106] Chromatography medium: Source 30 Q
[0107] Column specification: 5ml
[0108] mobile phase:
[0109] A 0.05M Na 2 HPO 4 -NaH 2 PO 4 , pH8.0
[0110] B 0.05M Na 2 HPO 4 -NaH 2 PO 4 , 0.4M NaCl, pH8.0
[0111] Flow rate: 5ml / min
[0112] Gradient: 1-15% 1 column vol...
Embodiment 3
[0135] Determination of biological activity (specific activity) of the modified product (see Chinese Pharmacopoeia 2005 edition: asparaginase)
[0136] Using the L-asparaginase standard as a control, the amount of catalytic substrate produced by the standard and the sample was determined, and then the specific activity (U / mg) of the sample was calculated. See Table 1.
[0137] Table 1: Biological activity of L-asparaginase and PEG-modified L-asparaginase
[0138] sample
[0139] The results show that the polyethylene glycol-modified L-asparaginase obtained by the invention has high biological activity.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com