Semaglutide liposome and preparation method thereof
A technology of samoglutide fat and samoglutide, which is applied in the field of medicine, can solve the problems of low bioavailability, strong side effects, instability of samoglutide, etc., and achieve the effect of reducing permeability, high encapsulation rate and avoiding aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] The preparation method of the samoglutide liposome provided by the invention can adopt the film dispersion method.
[0064] The preparation method of the samoglutide liposome provided by the invention comprises the following steps: after taking phospholipids and cholesterol and dissolving them in chloroform, removing the chloroform by rotary evaporation, adding PBS buffer solution containing samoglutide, and mixing with pharmaceutically acceptable The aqueous solution of the lyoprotectant is mixed and freeze-dried to obtain the product.
[0065] The particle size of the samorutate liposome prepared by the film dispersion method is 50nm-200nm.
[0066] The mass ratio of phospholipids, samoglutide, cholesterol and pharmaceutically acceptable cryoprotectant in the PBS buffer containing samoglutide prepared by the thin film dispersion method is (0.001~5): (0.1~ 500): (0.01~50): (0.01~100).
[0067] The molar-volume concentration of phosphate is 0.01 mol / L, and the pH valu...
Embodiment 1
[0100] Embodiment 1 film dispersion method prepares Samolutai liposome
[0101] Weigh each component:
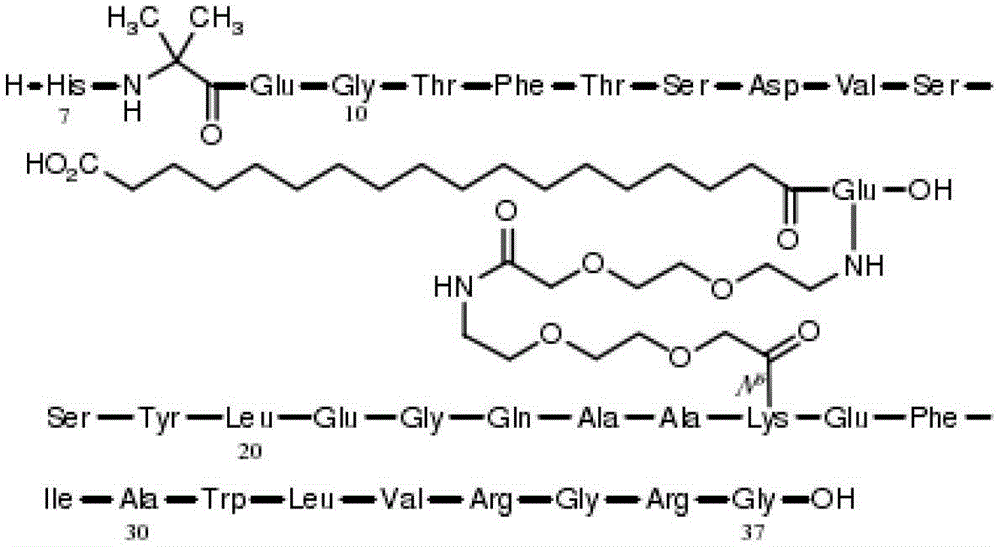
[0102] Samorutai
7mg
Dipalmitoylphosphatidylcholine (DPPC)
700mg
cholesterol
70mg
70mg
[0103] Take 700mg of dipalmitoylphosphatidylcholine (DPPC) and 70mg of cholesterol in a round bottom flask, add 10mL of chloroform and stir to dissolve, then remove the chloroform by vacuum evaporation, and form a uniform and transparent film on the wall; under the protection of nitrogen, in Add 200 μL of isotonic PBS buffer solution (pH6.5, 0.01mol / L phosphate) into the round bottom flask, containing 7 mg of samoglutide, stir for 4 minutes, and place it at 25 °C for 2 hours to make the film fully swell; Stir at 25°C for 2 hours to obtain a large unilamellar liposome suspension; pass the resulting large unilamellar liposome suspension through a high-pressure milk homogenizer twice, and filter through a 0.20 μm microporous me...
Embodiment 2
[0105] Embodiment 2 thin film dispersion method prepares Samolutai liposome
[0106] Weigh each component:
[0107] Samorutai
70mg
Dipalmitoylphosphatidylcholine (DPPC)
2g
cholesterol
200mg
100mg
[0108] Take 2 g of dipalmitoylphosphatidylcholine (DPPC) and 200 mg of cholesterol in a round-bottomed flask, add 50 mL of chloroform and stir to dissolve, remove the chloroform by vacuum evaporation, and form a uniform and transparent film on the wall; under the protection of nitrogen, in Add 2mL of isotonic PBS buffer solution (pH6.5, 0.01mol / L phosphate) into the round bottom flask, containing 70mg of samoglutide, stir for 4min, and place it at 25°C for 2 hours to make the film fully swell; Stir at 25°C for 2 hours to obtain a large unilamellar liposome suspension; pass the obtained liposome suspension twice through a high-pressure homogenizer, and filter through a 0.20 μm microporous filter membrane to obtain a smal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com