Synthesis method of semaglutide
A synthetic method, the technology of semaglutide, applied in the field of drug synthesis, to achieve the effect of reducing missing peptides, reducing the difficulty of synthesis, and increasing the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A kind of synthetic method of semaglutide, such as Figure 4 shown, including the following steps:
[0046] 1.1 Synthesis of the first peptide resin
[0047] Weigh 100 g of 2-CTC resin with a substitution degree of 0.993 mmol / g, add it to a solid-phase reaction vessel, wash it twice with DCM, add DCM to swell for 30 min, and after the swelling is complete, the resin is decompressed and dried for use.
[0048] Fmoc-Gly-OH (8.92g, 30mmol) and DIEA (38.77g, 300mmol) were dissolved in DCM (500ml), and added to the above-mentioned 2-CTC resin to start the synthesis of Fmoc-Gly-resin after the dissolution and clarification, the reaction temperature Controlled at 25-30°C, reacted for 3h. After the reaction was complete, the resin was washed twice with DCM, 500ml each time. Then add methanol / DCM=1 / 4 (500) and DIEA (129.13g, 1000mmol) to the resin, capping at 25-30°C for 1h, wash the resin twice with DCM after capping, 500ml each time, add methanol to shrink the resin and dr...
Embodiment 2
[0074] The difference between this example and Example 1 is that the condensing agent system in Example 1 is changed to DIC+HOAt, and 97.89 g of semaglutide with a purity of 81.12% of crude semaglutide is obtained by the same scale and method .
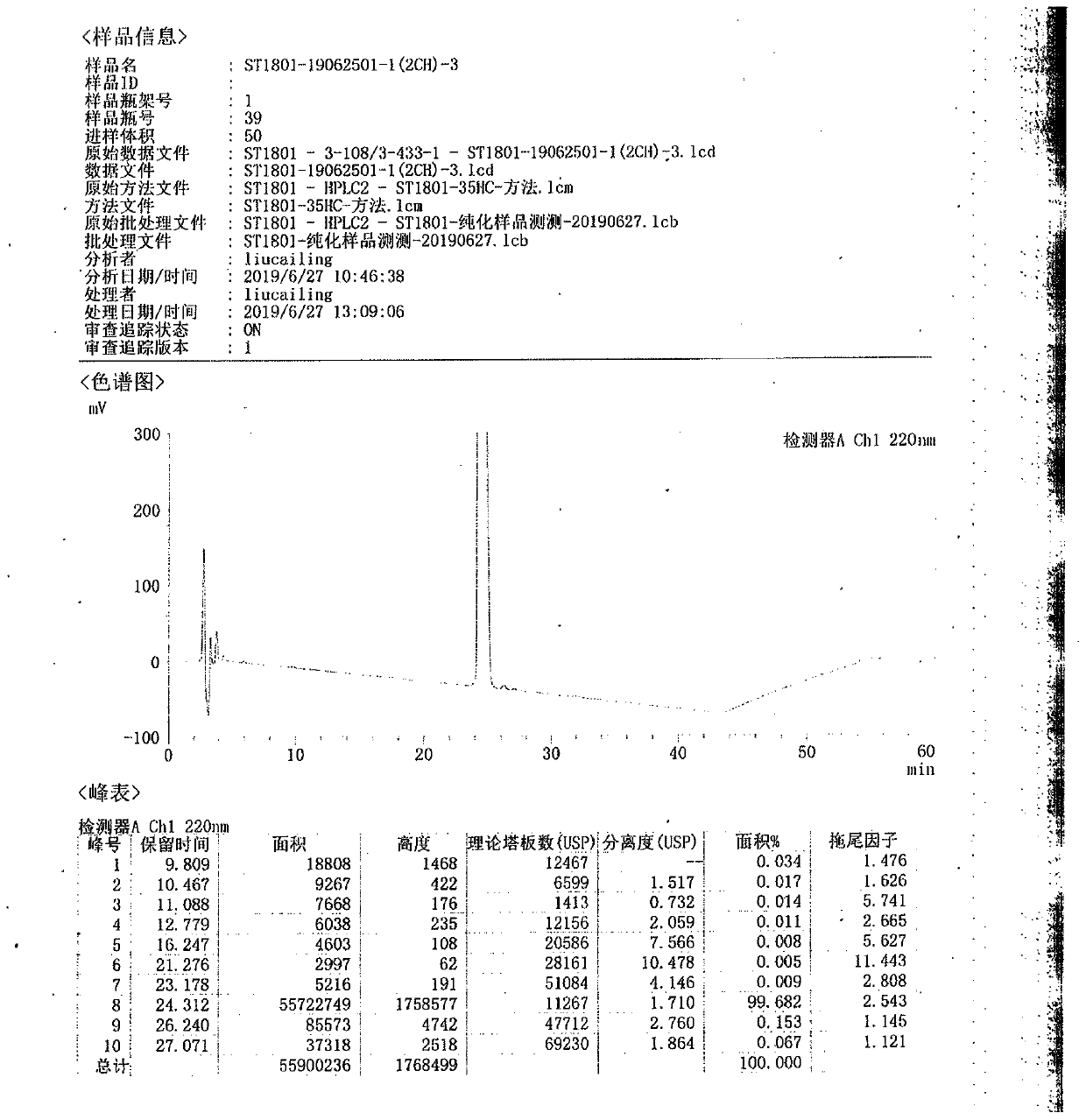
[0075] The pure product of semaglutide obtained by the same purification method in Example 1 is as follows: figure 2 As shown, the purity was 99.682%, and 1.12 g of pure semaglutide was obtained after purification of 2 g of crude semaglutide, with a yield of 56.15%.
Embodiment 3
[0077] The difference between this example and Example 1 is that the condensing agent system was changed to DIC+Cl-HOBt, and 106.23 g of semaglutide crude peptide with a purity of 83.12% was obtained by the same scale and method.
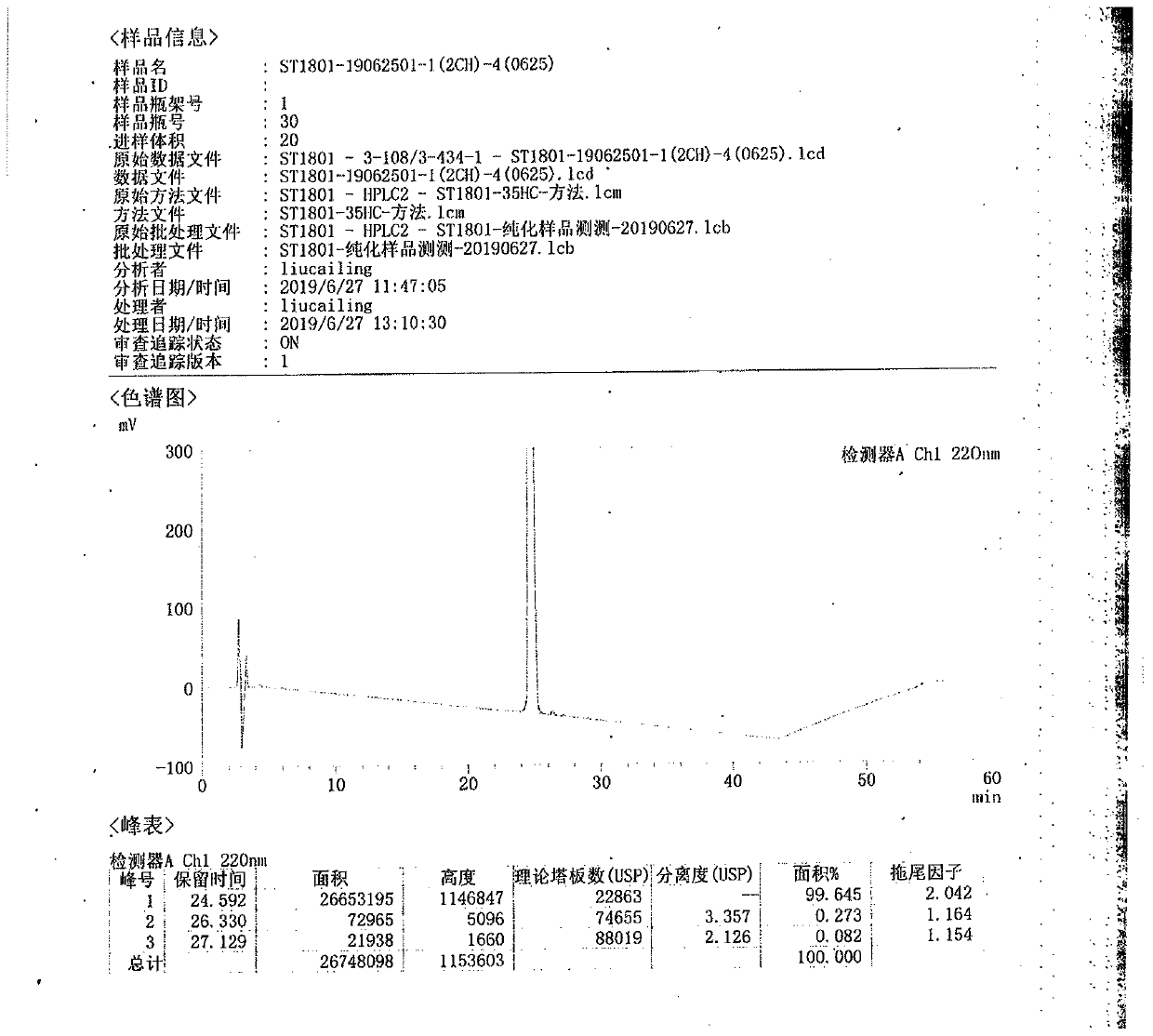
[0078] The pure product of semaglutide obtained by the same purification method in Example 1 is as follows: image 3 As shown, the purity is 99.985%, and 1.07 g of pure semaglutide is obtained after purification of 2 g of crude semaglutide, with a yield of 53.60%.
[0079] Some abbreviations used in the present invention have the following meanings:
[0080]
[0081]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com