Purification method of semaglutide
A technology of semaglutide, purification method, applied in the field of peptide purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Sample pretreatment:
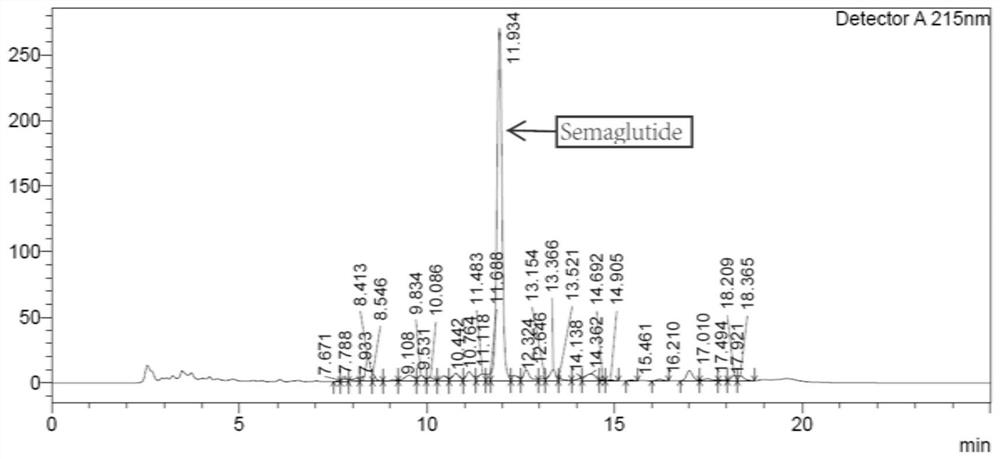
[0034] Take 2.0 g of crude semaglutide (containing about 0.8 g of semaglutide) sample and dissolve it in 1% ammonia solution. After completely dissolving, adjust the pH of the solution to 7.5 with phosphoric acid, and then bathe in water at 45°C to 50°C for 1 ~2h, filter with a 0.45um filter membrane, and collect the filtrate as an aqueous solution of crude semaglutide for future use.
[0035] (2) One-step purification:
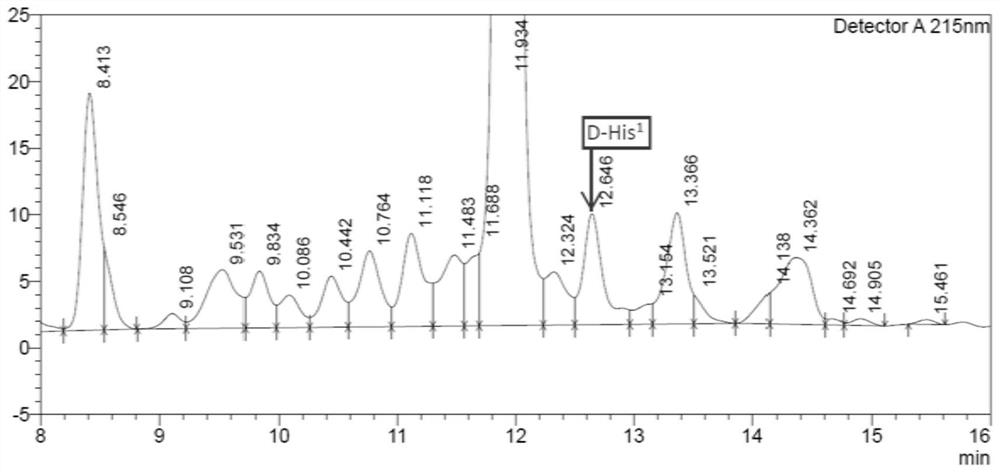
[0036] One-step purification of crude semaglutide aqueous solution, chromatographic conditions: Preparative chromatographic column: octaalkyl bonded silica gel column, chromatographic column specification is 30*250mm. Mobile phase A: 20mM / L ammonium chloride, aqueous solution adjusted to pH=7 with ammonia water, mobile phase B: acetonitrile; detection wavelength is 230nm, flow rate is 25ml / min, sample volume is 0.8g, elution gradient is: 0~ 5min: B% is 10%-26%; 5-120min: B% is 26%-46%.
[0037] Collect semaglutide fraction...
Embodiment 2
[0047] (1) Sample pretreatment:
[0048] Take 2.7g of crude semaglutide (containing about 1.08g of semaglutide) sample and dissolve it in 5% ammonia solution. After completely dissolving, use phosphoric acid to adjust the pH of the solution to 9, and then bathe in water at 45°C-50°C for 1 ~2h, filter with a 0.45um filter membrane, and collect the filtrate as an aqueous solution of crude semaglutide for future use.
[0049] (2) One-step purification:
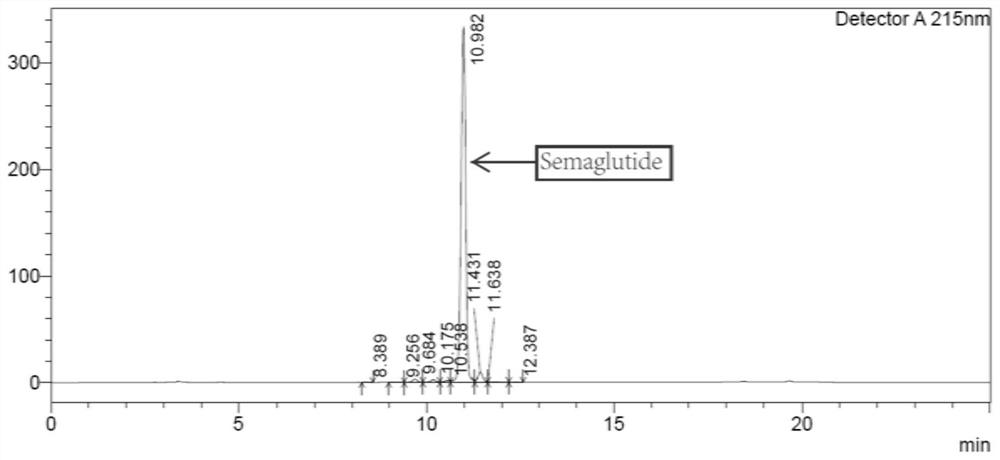
[0050] One-step purification of crude semaglutide aqueous solution, chromatographic conditions: Preparative chromatographic column: octaalkyl bonded silica gel column, chromatographic column specification is 30*250mm. Mobile phase A: 100mM / L ammonium chloride, ammonia water to adjust pH=9 aqueous solution, mobile phase B: acetonitrile; detection wavelength is 230nm, flow rate is 25ml / min, sample volume is 1.08g, elution gradient is: 0~ 5min: B% is 10%-26%; 5-120min: B% is 26%-46%.
[0051] Collect semaglutide fraction samples ...
Embodiment 3
[0061] (1) Sample pretreatment:
[0062] Take 2.3g of crude semaglutide (containing about 0.92g of semaglutide) sample and dissolve it in 2% ammonia solution. After completely dissolving, adjust the pH of the solution to 8 with phosphoric acid, and then bathe in water at 45°C-50°C for 1 ~2h, filter with a 0.45um filter membrane, and collect the filtrate as an aqueous solution of crude semaglutide for future use.
[0063] (2) One-step purification:
[0064] One-step purification of crude semaglutide aqueous solution, chromatographic conditions: Preparative chromatographic column: octaalkyl bonded silica gel column, chromatographic column specification is 30*250mm. Mobile phase A: 50mM / L ammonium chloride, aqueous solution adjusted to pH=8 with ammonia water, mobile phase B: acetonitrile; detection wavelength is 230nm, flow rate is 25ml / min, sample volume is 0.92g, elution gradient is: 0~ 5min: B% is 10%-26%; 5-120min: B% is 26%-46%.
[0065] Collect semaglutide fraction samp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com