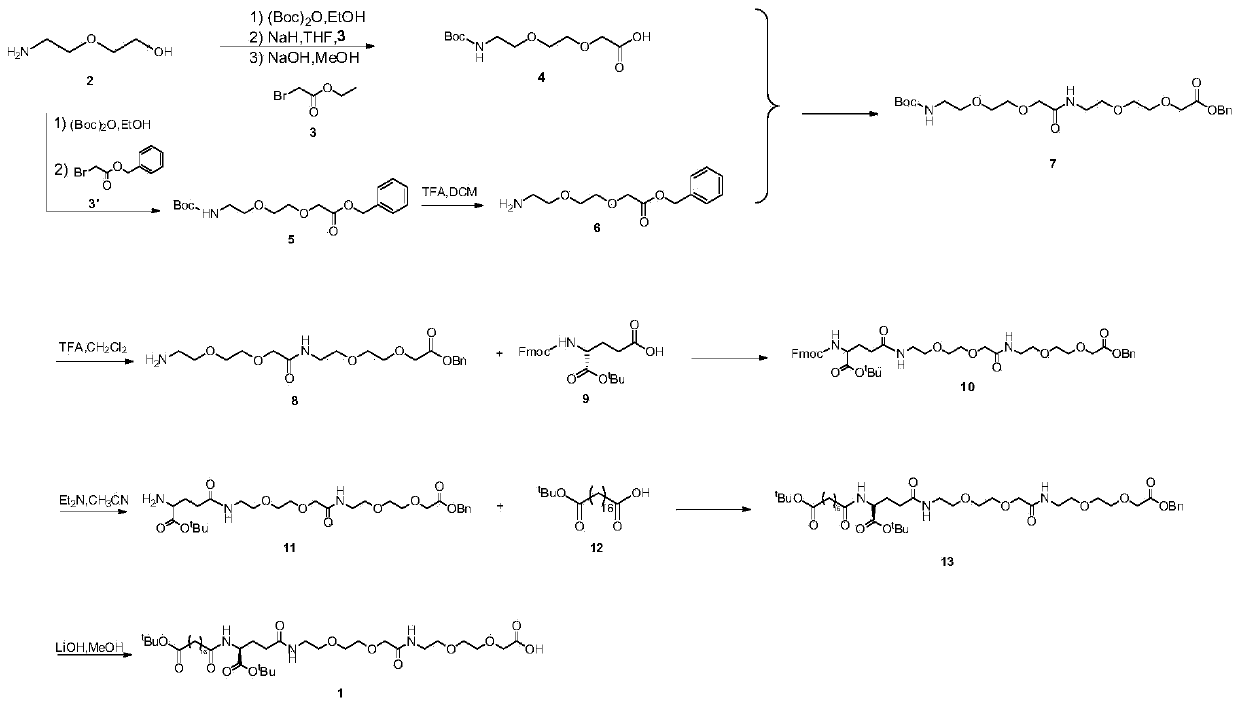
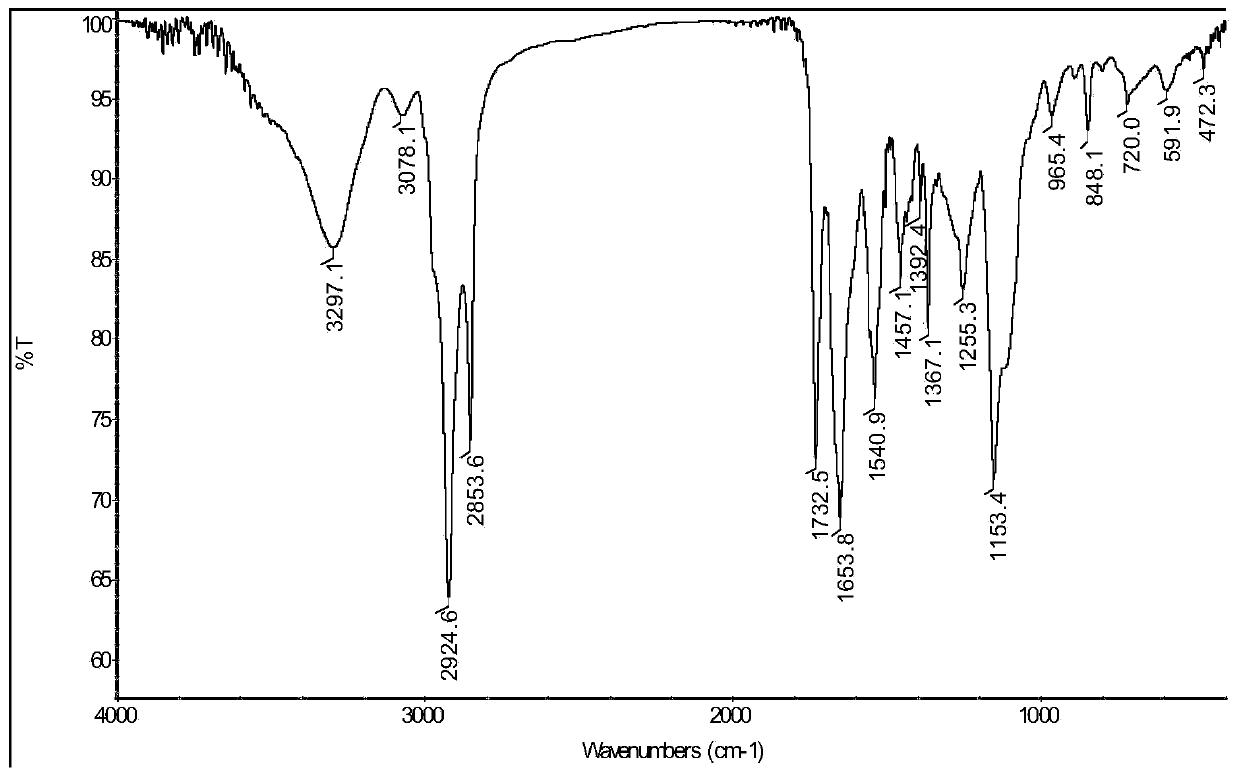
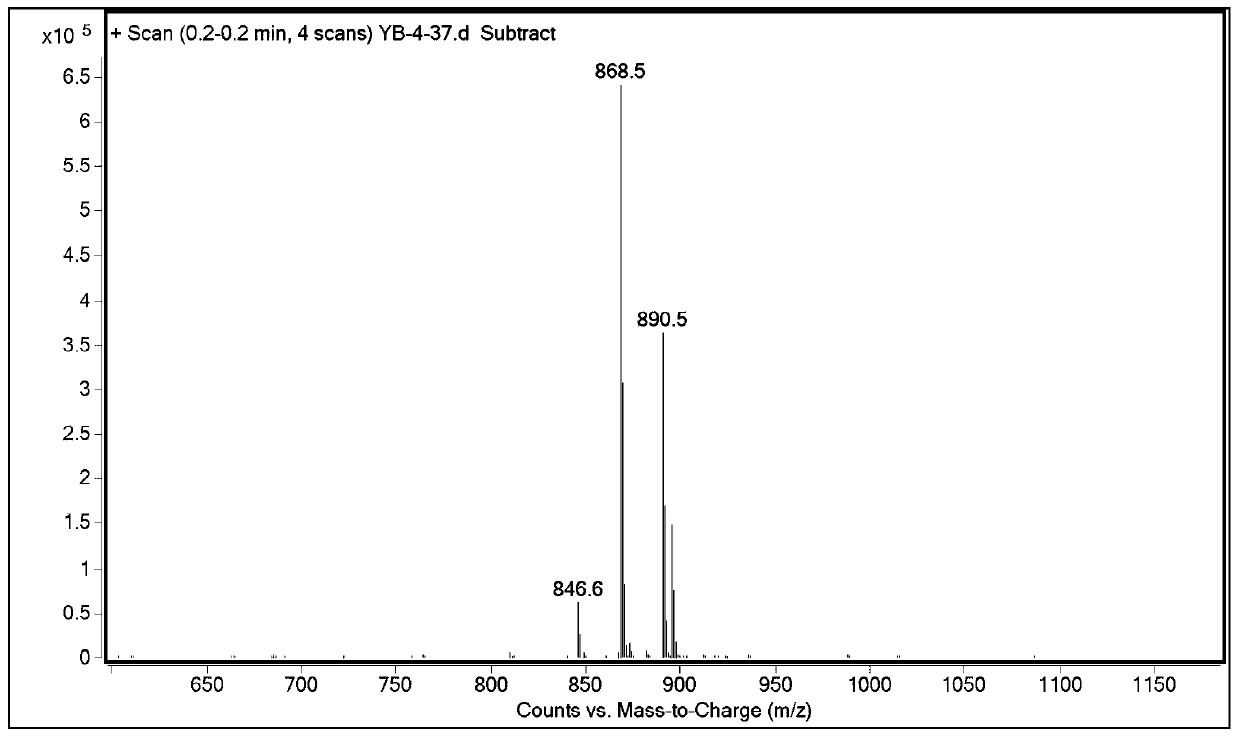
Method for preparing semaglutide side chain by liquid phase method
A side chain and aliphatic chain technology, applied in the field of liquid phase synthesis of side chains, can solve problems such as reducing renal clearance rate, and achieve the effect of low cost and wide selection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0078] One Pot Preparation
[0079] Dissolve 150mL of ethanol solution with 2-(2-aminoethoxy)ethanol 2 as the starting material (100mmol, 10.5g), add di-tert-butyl dicarbonate (100mmol, 23mL) dropwise under ice bath conditions, and remove the ice bath, react at room temperature for 2 hours, remove the ethanol solvent by distillation under reduced pressure; THF redissolved, add NaH (1.2-1.5eq) at 0~-10°C, keep stirring at this temperature for half an hour, and distill ethyl bromoacetate 3 (1.2-1.5eq ) slowly drop into the reaction solution, remove the ice bath after the dropwise addition, and stir overnight at room temperature. Add MeOH to the reaction system so that MeOH:THF=1:1, the reaction solution is yellow and clear, weigh solid NaOH (1-2.2eq) into the reaction solution, heat to reflux, and react for 2h. After the reaction was completed, the solvent was distilled off under reduced pressure, the residue was redissolved in water, extracted twice with ethyl acetate, the a...
Embodiment 1-2
[0081] preparation.
[0082] (1) Dissolve 150mL of ethanol solution with 2-(2-aminoethoxy)ethanol 2 as the starting material (100mmol, 10.5g), and add di-tert-butyl dicarbonate (100mmol, 23mL) dropwise under ice bath conditions , removed the ice bath, reacted at room temperature for 2h, distilled off the ethanol solvent under reduced pressure; THF redissolved, added NaH (1.2-1.5eq) at 0~-10°C, kept stirring at this temperature for half an hour, and distilled benzyl bromoacetate 3'( 1.2-1.5eq) slowly drop into the reaction solution, remove the ice bath after the dropwise addition, transfer to a 50°C oil bath and stir overnight. TLC monitoring reaction (CH 2 Cl 2 :EA=10:1). After the reaction was complete, the solvent was distilled off under reduced pressure, and the residue was redissolved in ethyl acetate, followed by extraction with 10% citric acid (75mL×2), saturated sodium bicarbonate solution (75mL×2), and washing with saturated brine (100mL). , the organic layer was...
Embodiment 1-3
[0085] preparation.
[0086] Weigh the above intermediate 4 (10mmol, 2.63g) and dissolve it in 25mL of dichloromethane, add N,N-diisopropylethylamine (3eq), EDCI (1.2eq), HOBT (1.2eq) in sequence, and stir After 30 min compound 6 (1.2 eq) was added and stirred overnight. After the reaction was complete, the solvent was distilled off under reduced pressure, and the residue was redissolved with ethyl acetate, extracted with 1M hydrochloric acid solution (50mL×2), saturated sodium bicarbonate solution (50mL×2), washed with saturated brine (75mL), The organic layer was collected and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain 7 as a yellow oil. Yield 79%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com