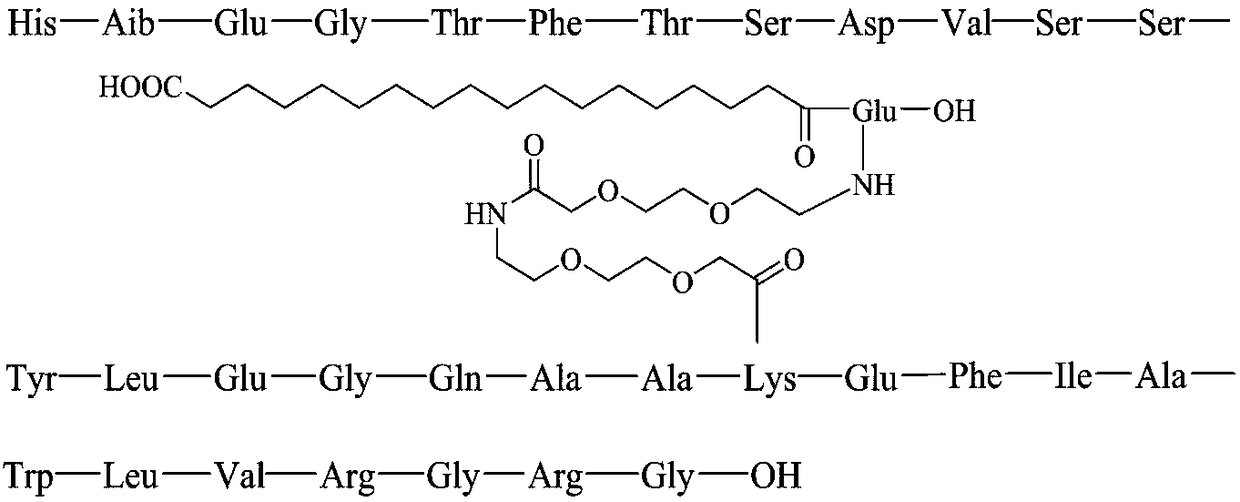
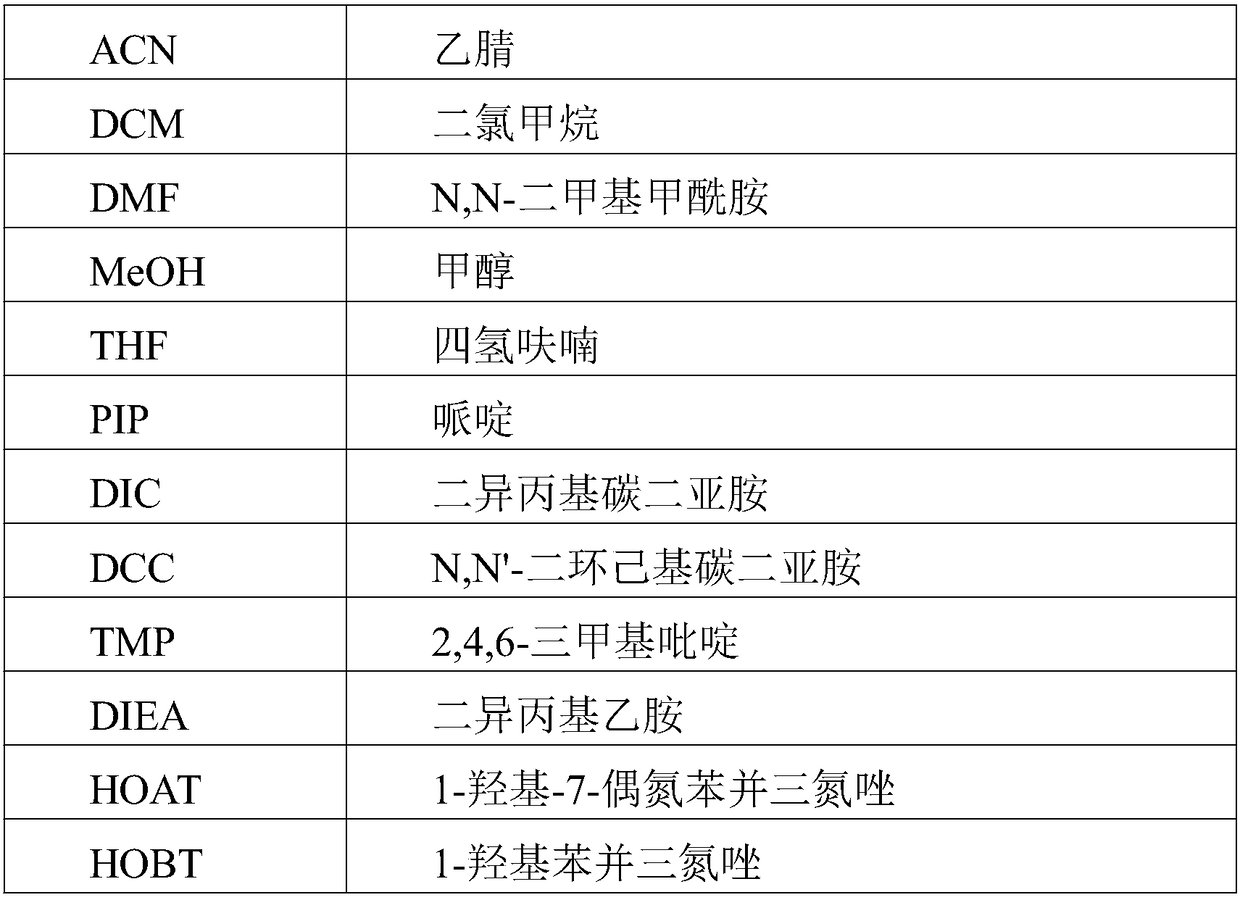
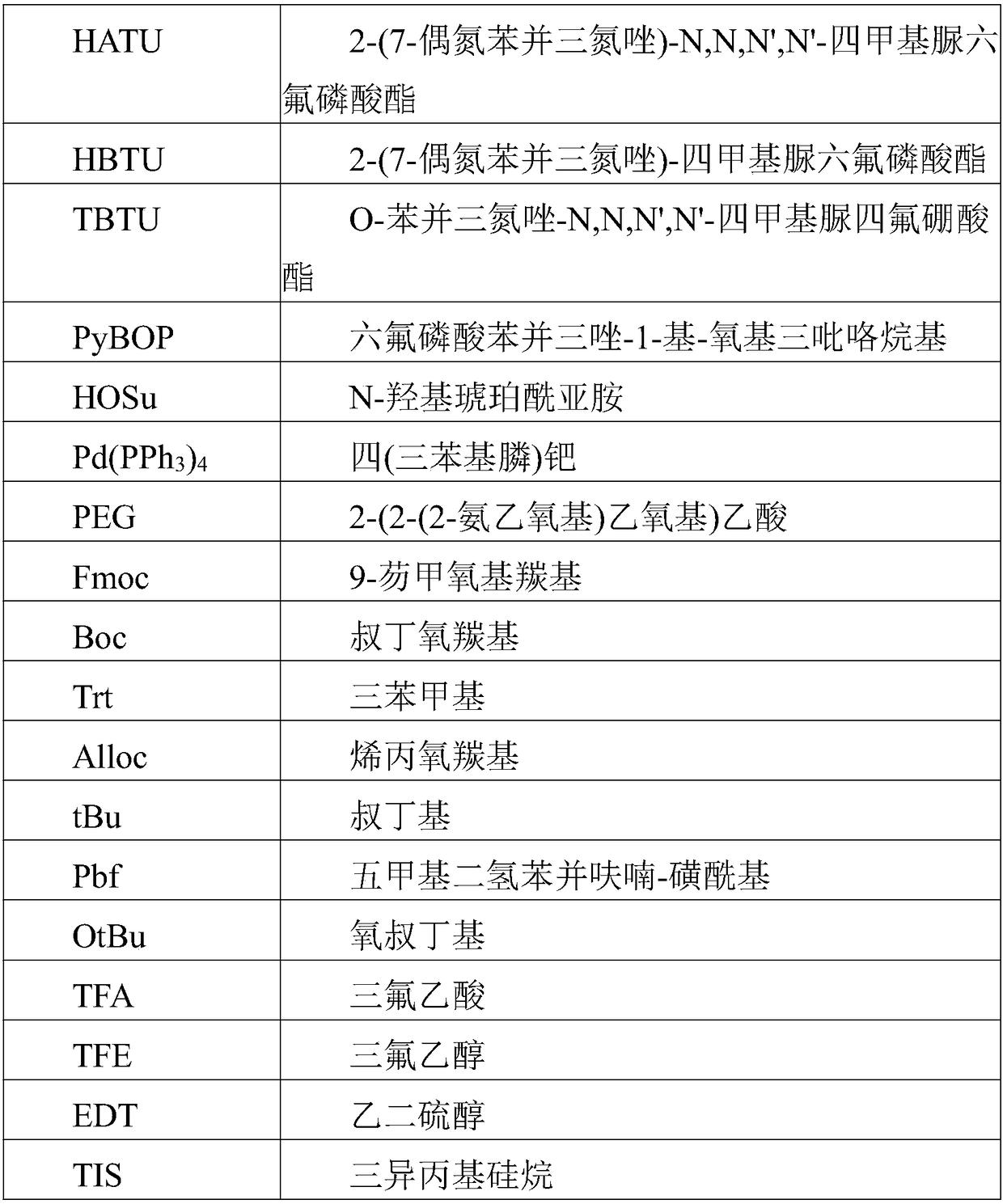
Method for preparing Semaglutide through solid and liquid combination
A semaglutide and synthetic method technology, applied in the field of polypeptide drug preparation, can solve the problems of product purity reduction, easy production of missing peptides, low efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Preparation of Alloc-Lys(PEG-PEG-γ-Glu(OtBu)-Monobutyl octadecanate)-OH:
[0058] 1) Resin swelling: Take 100g of CTC resin with a substitution degree of 0.5mmol / g, add 700ml of DCM or DMF to swell the resin for 0.5h, drain the solvent, wash the resin twice with DMF, and drain the solvent.
[0059]2) Preparation of Alloc-Lys(Fmoc)-resin: a) Mix Alloc-Lys(Fmoc)-OH, DIEA and resin according to the molar mass ratio of 3:6:1, and react at 25°C for 2h , to obtain Alloc-Lys(Fmoc)-resin; b) add a mixed solution of MeOH, DMF and DIEA to the resin, react at 10-30° C. for 30 min, and block the resin.
[0060] 3) Removal of Fmoc protecting group: add 20% PIP-DMF solution at 25°C to remove Fmoc protection twice: remove Fmoc protection for the first time and remove Fmoc protection for the second time, then wash the resin with DMF When the pH reaches 7, the solvent is drained; the reaction time for the first removal of Fmoc protection is 5 minutes, and the reaction time for the seco...
Embodiment 2
[0066] Liquid phase synthesis of dipeptide, tripeptide and tetrapeptide fragments:
[0067] 1) Preparation of Fmoc-Arg(Pbf)-Gly-OH:
[0068] a) Synthesis of Fmoc-Arg(Pbf)-OSu: Dissolve 0.2mol Fmoc-Arg(Pbf)-OH and 0.24mol HOSu in 0.4L THF, put in ice-water bath; dissolve 0.24mol DCC in 0.2L THF, and Add dropwise to the mixed solution in the previous step, continue to react for 1 h after the dropwise addition, then raise the temperature to 25°C and continue to react for 3 h; filter the reaction solution, evaporate to dryness, add DCM to dissolve and filter, and evaporate to dryness; add ethyl acetate to dissolve The solid was dissolved and recrystallized to obtain Fmoc-Arg(Pbf)-OSu.
[0069] b) Synthesis of Fmoc-Arg(Pbf)-Gly-OH: 0.15mol H-Gly-OH and 0.15mol Na 2 CO 3 Dissolve in 0.2L 50% THF-H 2 O solution to obtain a mixed solution; 0.1 mol Fmoc-Arg(Pbf)-OSu prepared in step a) was dissolved in THF, and added dropwise to the mixed solution in the previous step, reacted over...
Embodiment 3
[0106] Preparation of semaglutide peptide resin:
[0107] 1) Resin swelling: Take 25g of CTC resin with a substitution degree of 0.4mmol / g, add 200ml of DCM or DMF to swell the resin for 0.5h, drain the solvent, wash the resin twice with DMF, and drain the solvent.
[0108] 2) Preparation of Fmoc-Arg(Pbf)-Gly-resin: a) Mix Fmoc-Arg(Pbf)-Gly-OH, DIEA and resin according to the molar mass ratio of 3:6:1, at 25°C Under the condition of reaction for 2h, Fmoc-Arg(Pbf)-Gly-resin is obtained; b) Add the mixed solution of MeOH, DMF and DIEA to the resin, react at 10-30°C for 30min, seal the resin, and then use The resin was washed twice with DMF and the solvent was drained.
[0109] 3) Removal of Fmoc protecting group: add 20% volume fraction of PIP-DMF solution to the resin, and perform two removal of Fmoc protection at 10-30°C: after the first removal of Fmoc protection and the second removal of Fmoc protection , and then wash the resin with DMF to pH 7; the first de-Fmoc protecti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com