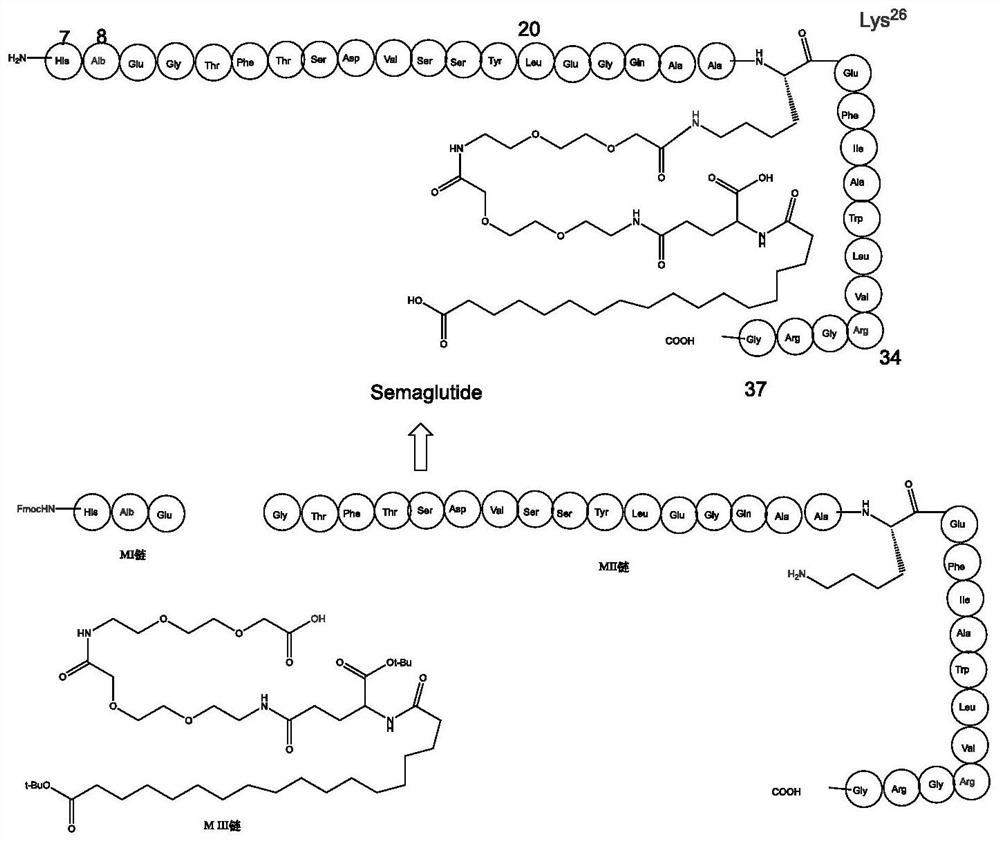
Method for preparing semaglutide by biochemical method
A biochemical and chemical technology, applied in the field of recombinant polypeptide preparation, can solve the problems of low yield, unfriendly environment and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
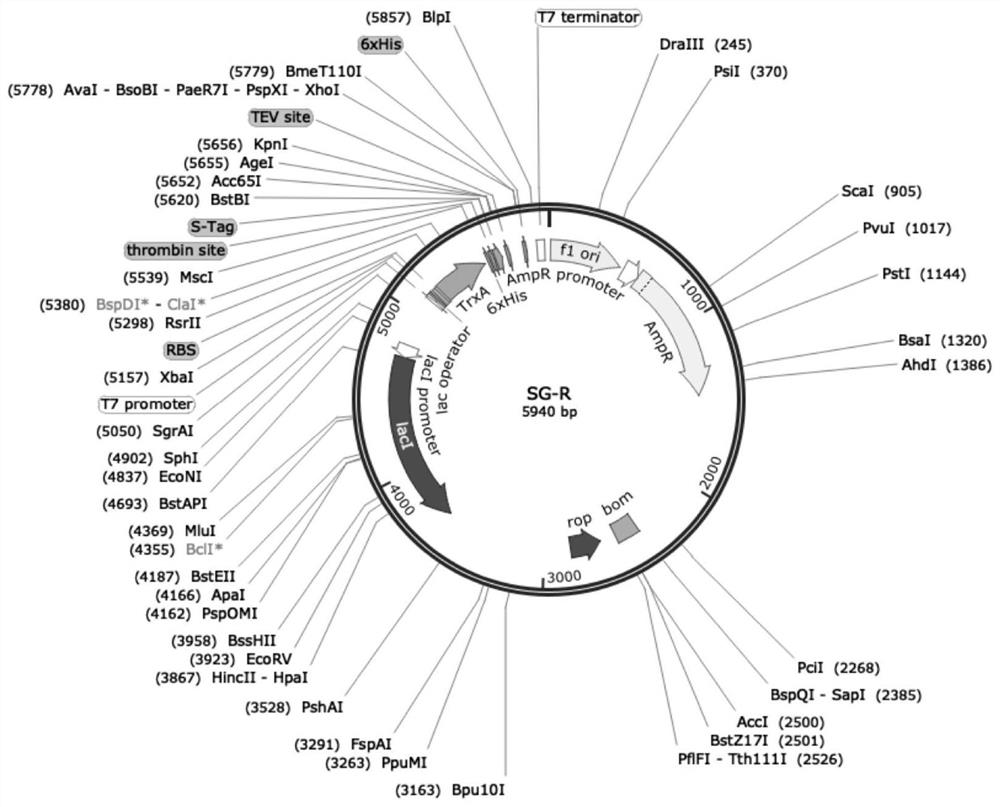
[0049] Construction of embodiment 1 fusion protein expression plasmid
[0050] Design the expression vector sequence (full sequence as shown in SEQ ID No.2), and send out the DNA sequence synthesis of the plasmid, which contains the coding gene, which contains the TrxA gene, TEV restriction site sequence and M II chain Sequence; After the plasmid was synthesized, PCR was used to confirm that the target gene of the fusion protein was synthesized correctly, and the upstream and downstream primers were designed F: 5'-TAATACGACTCACTATAGGG-3'R: 5'-GCTAGTTATTGCTCAGCGG-3'. The PCR conditions are: 98°C for 5min, 98°C for 30s, 56°C for 90s, 72°C for 90s, 36 cycles; PCR amplification system: template 1.5 μL, upstream and downstream primers 1.5 μL, sterilized double distilled water 20.5 μL, PrimerSTAR Mix 25 μL; After the PCR product is confirmed to run on the gel, send it to the detection sequence to confirm that the DNA sequence of the fusion protein is correct, and then construct the ...
Embodiment 2
[0051] Embodiment 2 Expression Engineering Bacteria Preparation and Positive Verification
[0052] Transform the constructed vector into the expression cell E.coli BL21(DE3) by electric shock for 1S, spread it on the LB plate containing kanamycin, put it in a 37°C incubator overnight, and grow a single colony Plasmid extraction and sequencing were carried out, and finally the recombinant engineering bacteria containing the esterase gene were confirmed to be positive engineering bacteria.
Embodiment 3
[0053] Expression and purification of embodiment 3 fusion protein
[0054] Transfer the seed liquid of the recombinant positive engineering strain of Example 2 to LB medium according to the inoculum size of 2%, cultivate it at 37°C until OD600=0.6, add 50 μl of 0.5mol / L IPTG, induce at 15°C for 16 hours, and collect by centrifugation For each 1 g of wet bacteria, resuspend in 10 mL of lysis buffer (50 mM Tris-HCl, 50 mM NaCl, 5% glycerol, pH 8.0, 17 μl (10 mg / mL PMSF)), ultrasonicate and centrifuge to obtain supernatant and precipitate. Collect the supernatant, 1:1 with nickel column adsorption buffer (10mM imidazole, 50mM NaCl, 50mM Tris-HCl pH8.0) (mixed), nickel column adsorption (prepacked column, commercially available), twice the column volume adsorption Buffer washing, 20mM imidazole buffer (20mM imidazole, 50mM NaCl, 50mM Tris-HCl pH8.0) was washed again to remove impurities. The elution buffer was used for elution of the target fusion protein (250mM imidazole, 50mM N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com