Method for purifying sermaglutide
A technology of semaglutide and semaglutide crude peptide, which is applied in the field of peptide purification, can solve the problems of low yield and low purity of semaglutide samples, and achieve the effect of improving purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
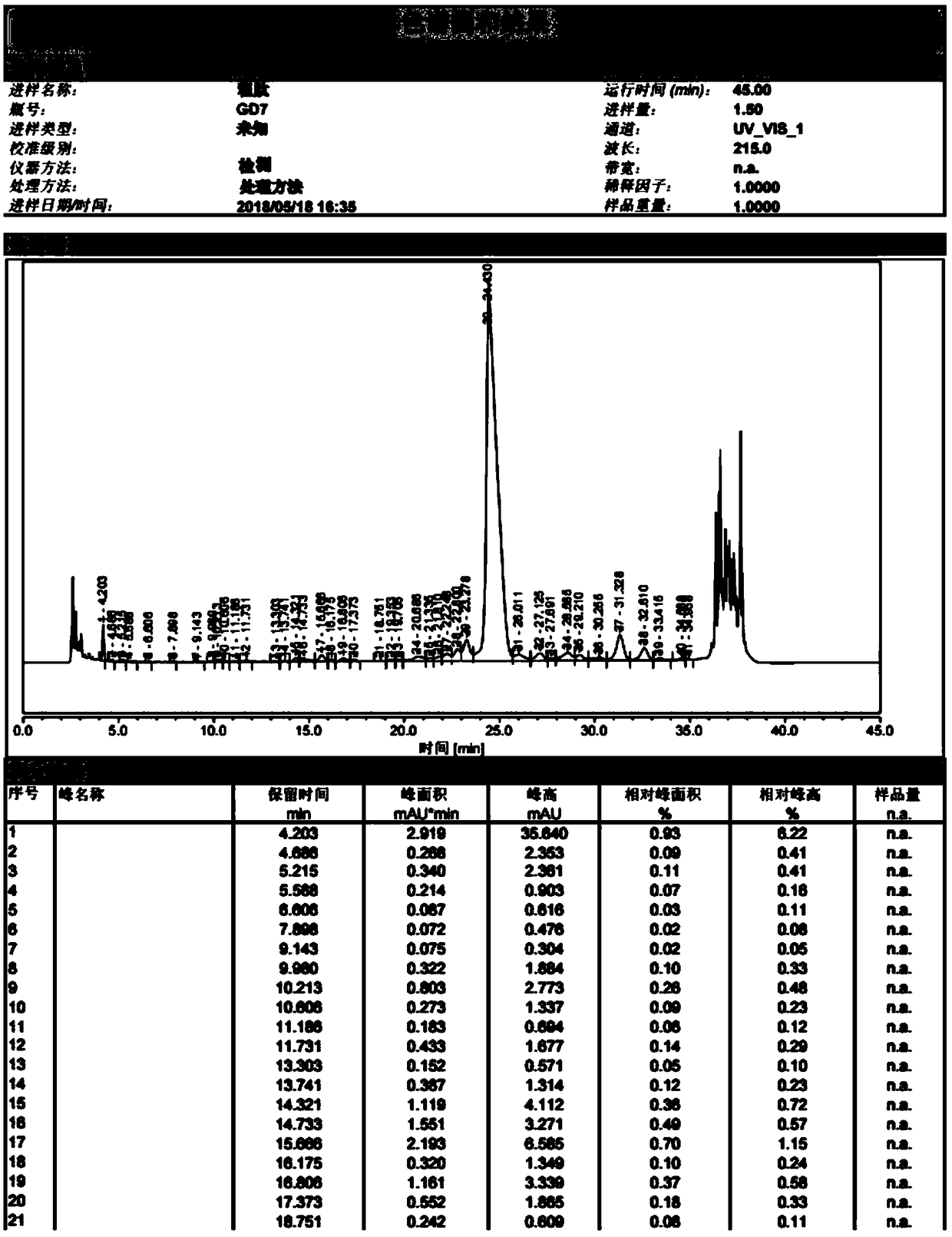
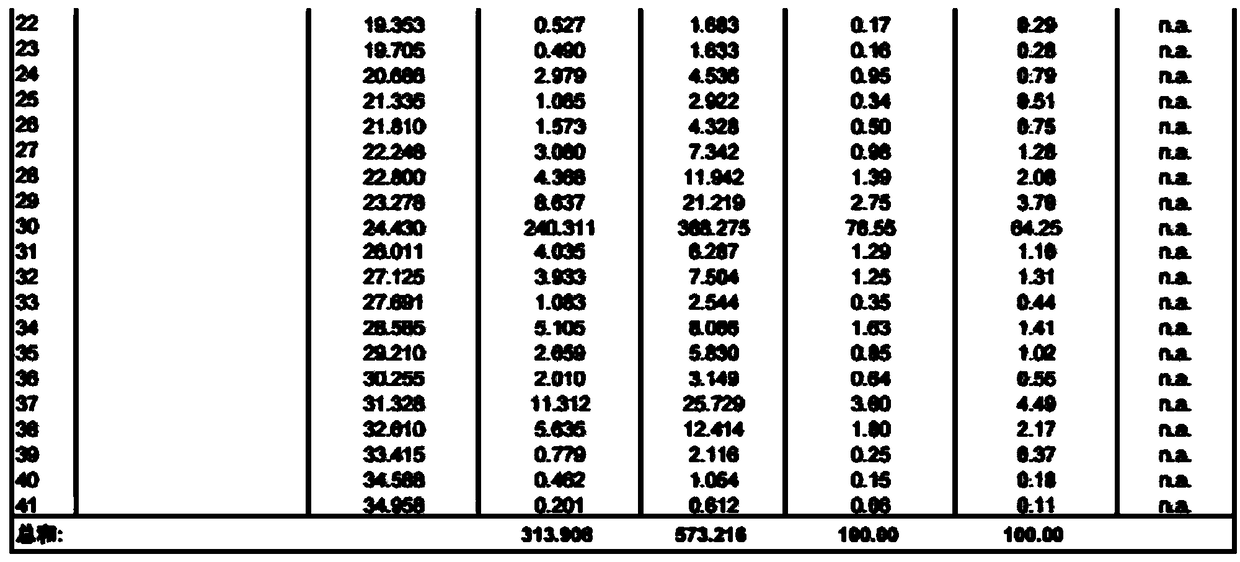
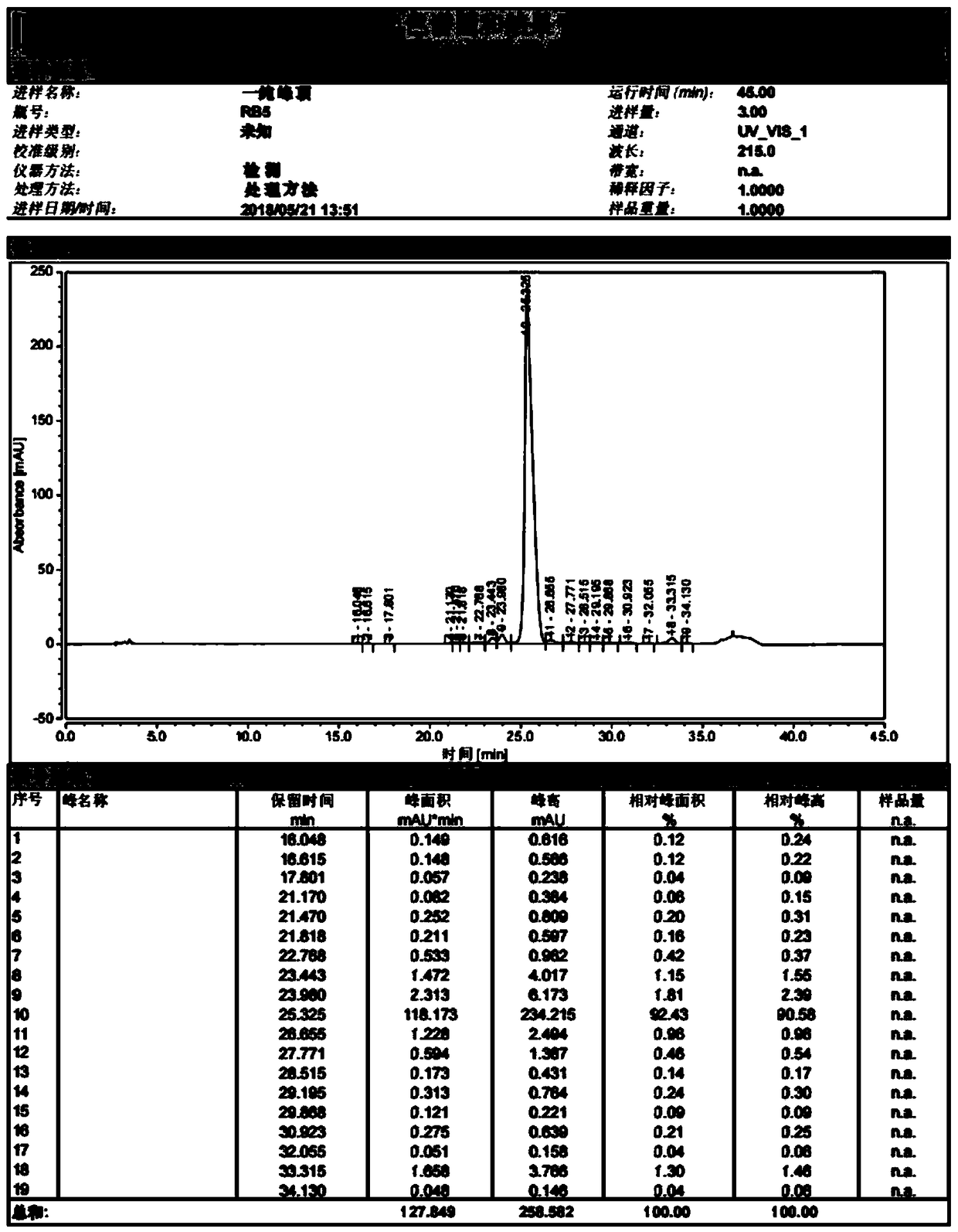
[0050] Take crude semaglutide
[0051] Sample treatment: Dissolve a sample containing 3 g of semaglutide crude peptide (crude peptide: 4.6 g) in aqueous acetonitrile, and filter it with a 0.22 μm filter membrane after complete dissolution. Collect the filtered semaglutide crude peptide aqueous solution for later use.
[0052] The first step of HPLC purification
[0053] Chromatographic conditions: chromatographic column with tetraalkylsilane bonded silica gel filler as stationary phase (30mm×250mm, 10μm); 0.2% phosphoric acid (take 1000ml water, add 2ml phosphoric acid, mix well, adjust the pH value to 2.3 with ammonia water) The mobile phase is A; acetonitrile is used as the mobile phase B; the flow rate is 20 mL per minute; the detection wavelength is 230 nm; the sample volume per needle is 0.6 g. The elution gradient in the following table was used for elution.
[0054]
[0055] Fractions of semaglutide samples with a purity greater than 95% were collected. Use a rot...
Embodiment 2
[0062] Take crude semaglutide
[0063] Sample treatment: Dissolve a sample containing 8g of semaglutide crude peptide (crude peptide: 12.2g) in aqueous acetonitrile, and filter through a 0.22μm filter membrane after complete dissolution. Collect the filtered crude peptide aqueous solution for later use.
[0064] The first step of HPLC purification
[0065] Chromatographic conditions: chromatographic column with tetraalkylsilane bonded silica gel filler as stationary phase (50mm×250mm, 10μm); 0.2% phosphoric acid (take 1000ml water, add 2ml phosphoric acid, mix well, adjust the pH value to 2.3 with ammonia water) The mobile phase is A; acetonitrile is used as the mobile phase B; the flow rate is 60 mL per minute; the detection wavelength is 230 nm; the loading amount is 1.6 g. The elution gradient in the following table was used for elution.
[0066]
[0067] Fractions of semaglutide samples with a purity greater than 95% were collected. Use a rotary evaporator with a wa...
Embodiment 3
[0073] Take crude semaglutide
[0074] Sample treatment: Dissolve a sample containing 32 g of crude semaglutide peptide (crude peptide: 48.8 g) in aqueous acetonitrile, and filter through a 0.22 μm filter membrane after complete dissolution. Collect the filtered crude peptide aqueous solution for later use.
[0075] The first step of HPLC purification
[0076] Chromatographic conditions: chromatographic column with tetraalkylsilane bonded silica gel filler as stationary phase (100mm×250mm, 10μm); 0.2% phosphoric acid (take 1000ml water, add 2ml phosphoric acid, mix well, adjust the pH value to 2.3 with ammonia water) Mobile phase A; acetonitrile as mobile phase B; flow rate of 200mL per minute; detection wavelength of 230nm; sample loading of 6.4g. The elution gradient in the table below was used for elution.
[0077]
[0078] Fractions of samples containing semaglutide were collected. Use a rotary evaporator with a water bath temperature of 30-35°C and a vacuum below -...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com