Main peptide chain of semaglutide and preparation method thereof
A semaglutide and peptide chain technology, applied in the field of semaglutide main peptide chain and its preparation, can solve the lack of high-purity and high-yield semaglutide main peptide preparation method, inclusion body dissolution and modification. Long renaturation time, unsuitable for large-scale production, etc., to reduce the renaturation process, reduce the cost of chemical reagents, and reduce impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
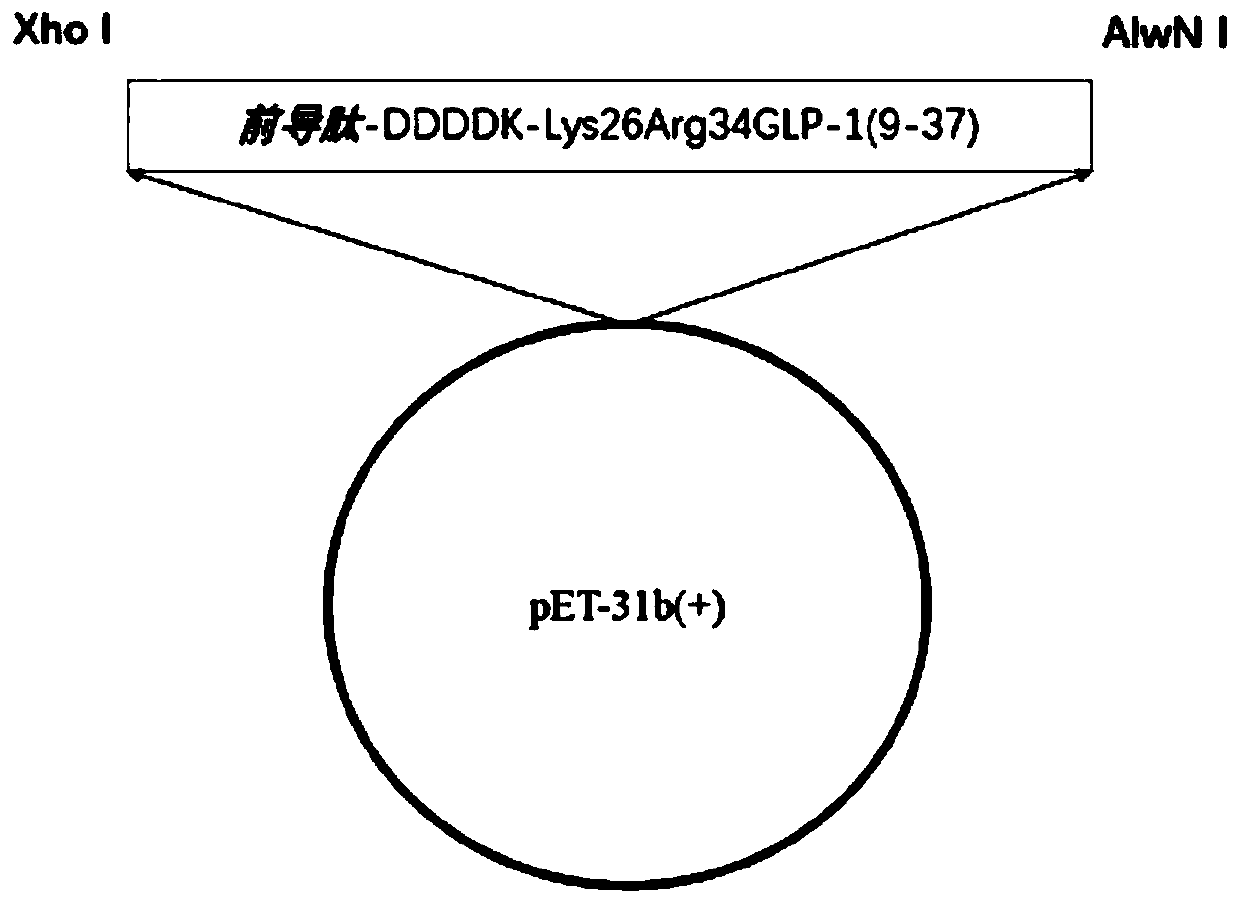
[0045] The recombinant protein of a semaglutide intermediate polypeptide of the present invention is characterized in that: the recombinant protein of the semaglutide intermediate polypeptide is a leader peptide-DDDDK-GLP-1(9-37), and the recombinant protein of the semaglutide intermediate polypeptide is The amino acid sequence of the recombinant protein of the marutide intermediate polypeptide is shown in SEQ ID NO.1;
[0046] In the preparation of semaglutide intermediates, the following leader peptides can be used:
[0047] (1) MFLKGDGYVQGIINFEGLHHLVALGLV; (2) KSI; (3) TrxA; (4) DsbA, (5) DsbC; (6) Sumo; (7) GST; (8) Intein; (9) KPSTYI.
[0048] A gene recombinant expression plasmid of the present invention contains the coding gene encoding the recombinant protein described in claim 1.
[0049] A kind of recombinant engineering bacterium comprising the gene recombination expression plasmid of the present invention adopts the gene recombination expression plasmid to transfe...
Embodiment 2
[0061] The difference between embodiment 2 and embodiment 1 is:
[0062] A kind of preparation method of semaglutide main peptide chain of the present invention comprises the following steps:
[0063] In step (6), the bacterium is subjected to high-pressure homogenization, the inclusion bodies are collected, and then the inclusion bodies are washed and denatured; the washed inclusion bodies are subjected to an alkaline condition with a pH of 14, according to the protein concentration of 30g / L inclusion body dissolution buffer was added to dissolve and denature inclusion bodies.
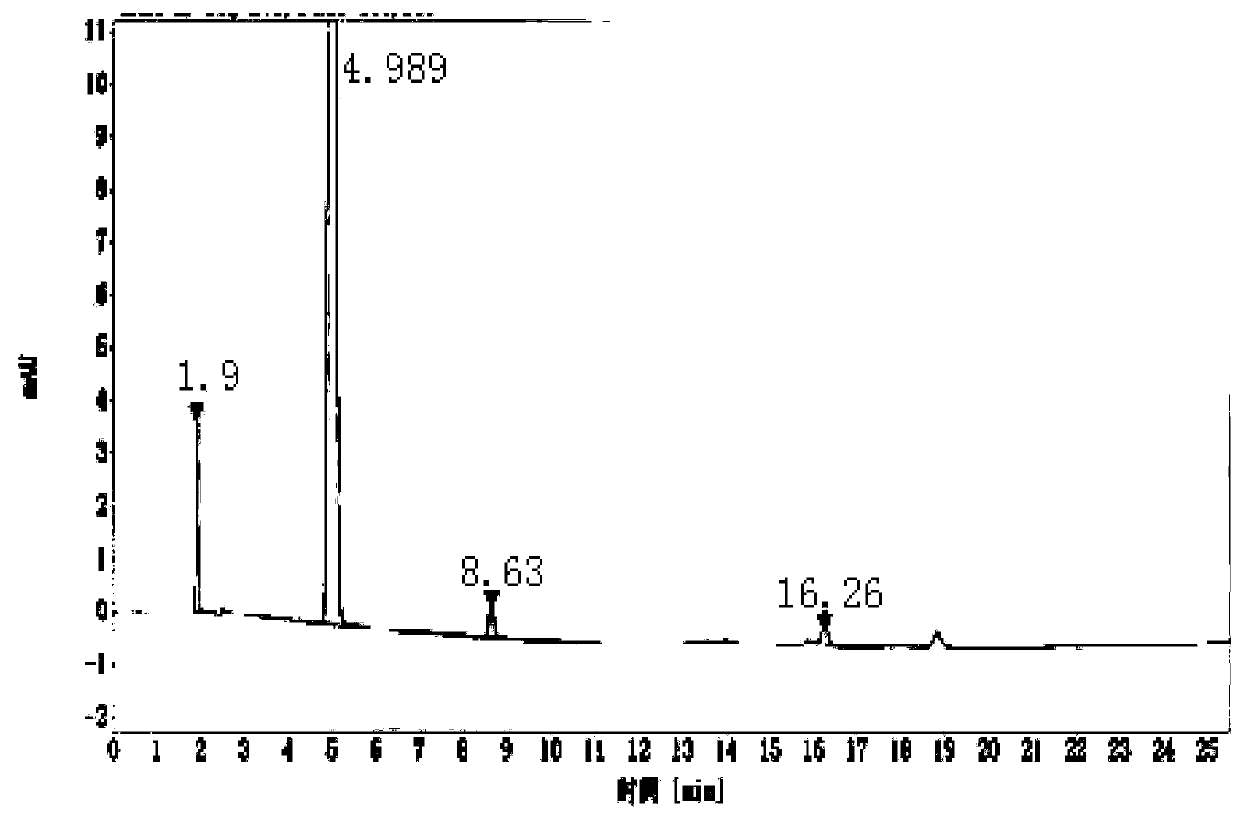
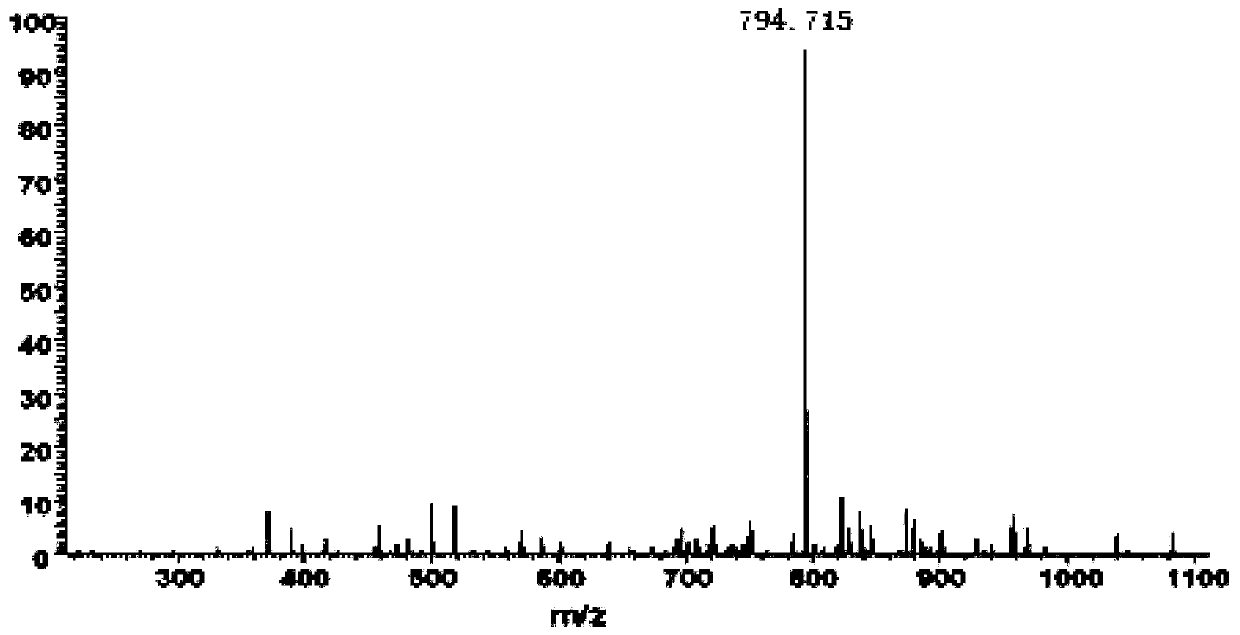
[0064] In step (7), the intermediate polypeptide GLP-1 (9-37) is obtained by enzymatic conversion, separation and purification; the specific method of the enzyme conversion, separation and purification is: the recombinant protein after step (6) renaturation The pH range of the solution described by enterokinase is 11.0; the recombinant protein after step (6) denaturation and renaturation is hydrolyze...
Embodiment 3
[0067] The difference between embodiment 3 and embodiment 1 is:
[0068] A kind of preparation method of semaglutide main peptide chain of the present invention comprises the following steps:
[0069] In step (6), the bacterium is subjected to high-pressure homogenization, the inclusion bodies are collected, and then the inclusion bodies are washed and refolded; the washed inclusion bodies are subjected to an alkaline condition with a pH of 12, according to the protein concentration of 5g / L inclusion body dissolution buffer was added to dissolve and denature inclusion bodies.
[0070] In step (7), the intermediate polypeptide GLP-1 (9-37) is obtained by enzymatic conversion, separation and purification; the specific method of the enzyme conversion, separation and purification is: the recombinant protein after step (6) renaturation The pH range of the solution described by enterokinase is 10.5; the recombinant protein after step (6) denaturation and renaturation can be obtaine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com