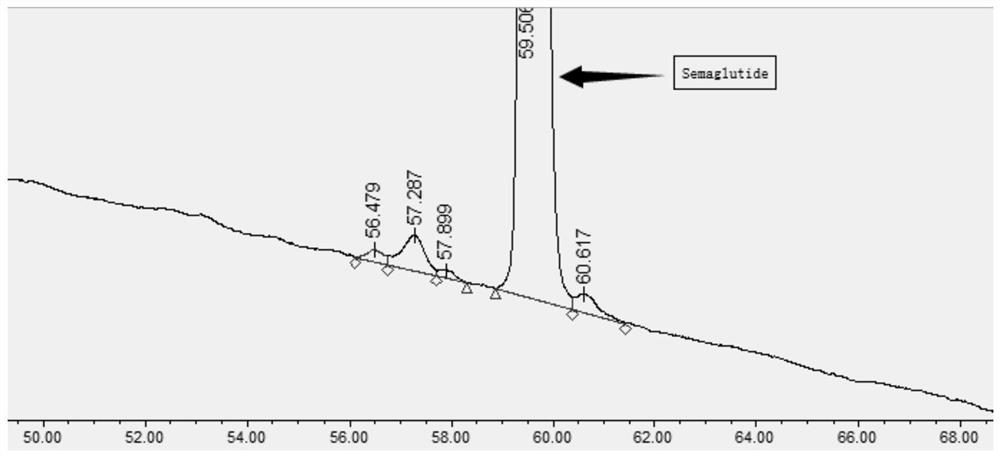
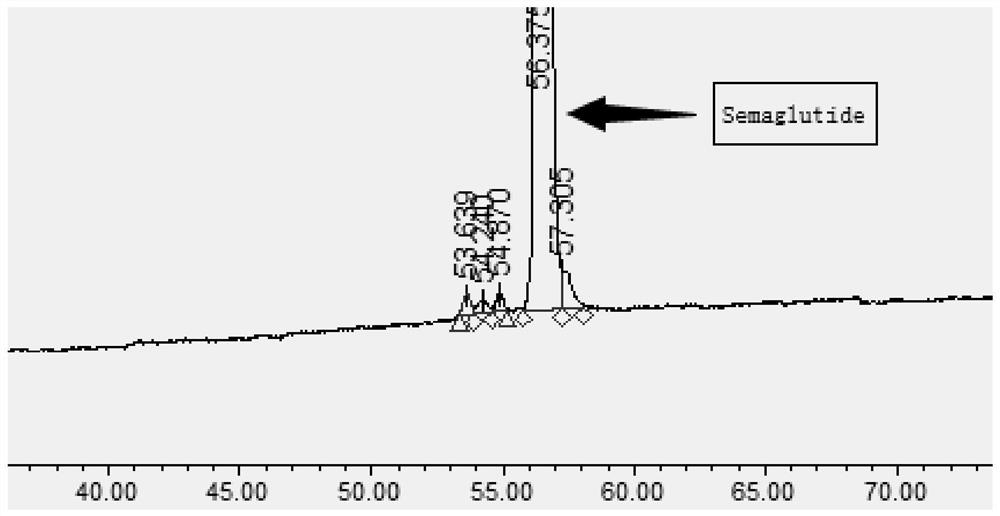
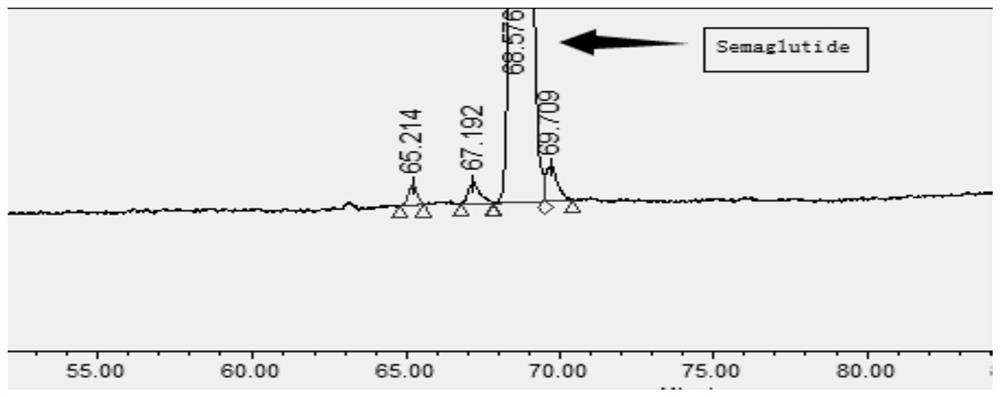
Ultra-high performance liquid chromatography analysis method of semaglutide
An ultra-high performance liquid chromatography and chromatographic analysis technology, which can be used in analytical materials, material separation, measurement devices, etc., and can solve problems such as unclear toxicological data, patient medication risks, and impact on the quality of semaglutide products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Chromatographic conditions
[0068]Instrument: ACQUITY CLASS-H (Wterse)
[0069] Chromatographic column: Chromatographic column 1: C8 column and C4 column in series
[0070] Column 2: C4 column and C18 column connected in series
[0071] Column 3: C8 column and C18 column connected in series
[0072] Mobile phase A: a mixed aqueous solution of 10mmol / L sodium perchlorate and 1mmol / L potassium hexafluorophosphate, adjust the pH to 3.0 with perchloric acid;
[0073] Mobile Phase B: Acetonitrile
[0074] Flow rate: 0.3ml / min
[0075] Column temperature: 45°C
[0076] Injection volume: 0.2μL
[0077] Detection wavelength: 214nm
[0078] Elution gradient: time: 0-120min, mobile phase B: 35-45%
[0079] The test was carried out with three different chromatographic columns connected in series, and 0.2ul of the test solution was accurately measured and injected into the chromatograph, and the chromatogram was recorded. The test results are shown in Table 1.
[0080] Ta...
Embodiment 2
[0085] Chromatographic conditions
[0086] Instrument: ACQUITY CLASS-H (Wterse)
[0087] Chromatographic column: C8 column and C4 column connected in series
[0088] Mobile phase A1: a mixed aqueous solution of 10mmol / L sodium perchlorate and 1mmol / L potassium hexafluorophosphate, adjust the pH to 3.0 with perchloric acid;
[0089] Mobile phase A2: 10mmol / L sodium perchlorate, adjust the pH to 3.0 with perchloric acid;
[0090] Mobile phase A3: a mixed aqueous solution of 10mmol / L sodium perchlorate, 1mmol / L potassium hexafluorophosphate, and 1mmol / L sodium trifluoroacetate, adjust the pH to 3.0 with perchloric acid;
[0091] Mobile Phase B: Acetonitrile
[0092] Flow rate: 0.3ml / min;
[0093] Column temperature: 50°C
[0094] Injection volume: 0.2μL
[0095] Detection wavelength: 214nm
[0096] Elution gradient: time: 0-120min, mobile phase: B: 35-45%;
[0097] The mobile phase A of the following three different ion-pairing solvents was tested respectively, and 0.2ul ...
Embodiment 3
[0103] Chromatographic conditions
[0104] Instrument: ACQUITY CLASS-H (Wterse)
[0105] Chromatographic column: C8 column and C4 column connected in series;
[0106] Mobile phase A: a mixed aqueous solution of 10mmol / L sodium perchlorate and 1mmol / L potassium hexafluorophosphate;
[0107] Mobile phase pH1: adjust the pH to 3.0 with perchloric acid;
[0108] Mobile phase pH2: adjust the pH to 2.0 with perchloric acid;
[0109] Mobile phase pPH3: adjust the pH to 4.0 with perchloric acid;
[0110] Mobile phase B: acetonitrile;
[0111] Flow rate: 0.3ml / min;
[0112] Column temperature: 55°C
[0113] Injection volume: 0.2μL
[0114] Detection wavelength: 214nm
[0115] Elution gradient: time: 0-60min, mobile phase B: 35-45%;
[0116] The following 3 different pHs were tested respectively, and 0.2ul of the test solution was accurately measured and injected into the chromatograph, and the chromatogram was recorded. The test results are shown in Table 3.
[0117] table 3 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| mobile phase | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com