Preparation method of Semaglutide
A technology of semaglutide and peptide chain, which is applied in the field of polypeptide drug preparation, can solve problems such as excessive use of amino acid raw materials, difficulty in amino acid condensation, and reduced product yield, so as to achieve extensive practical value and application prospects, and improve solubility problems , the effect of improving the recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
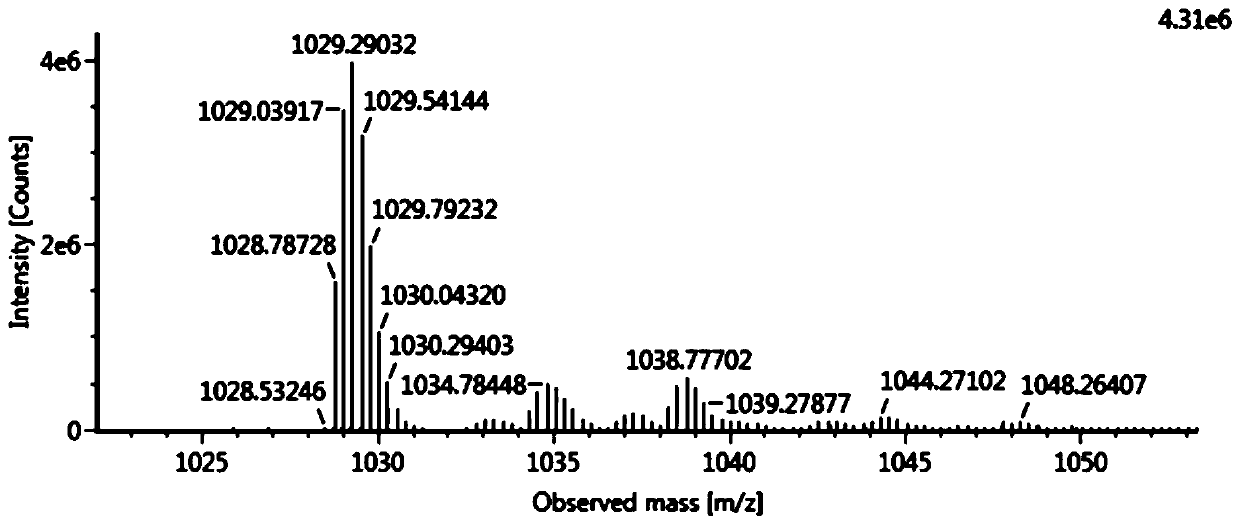
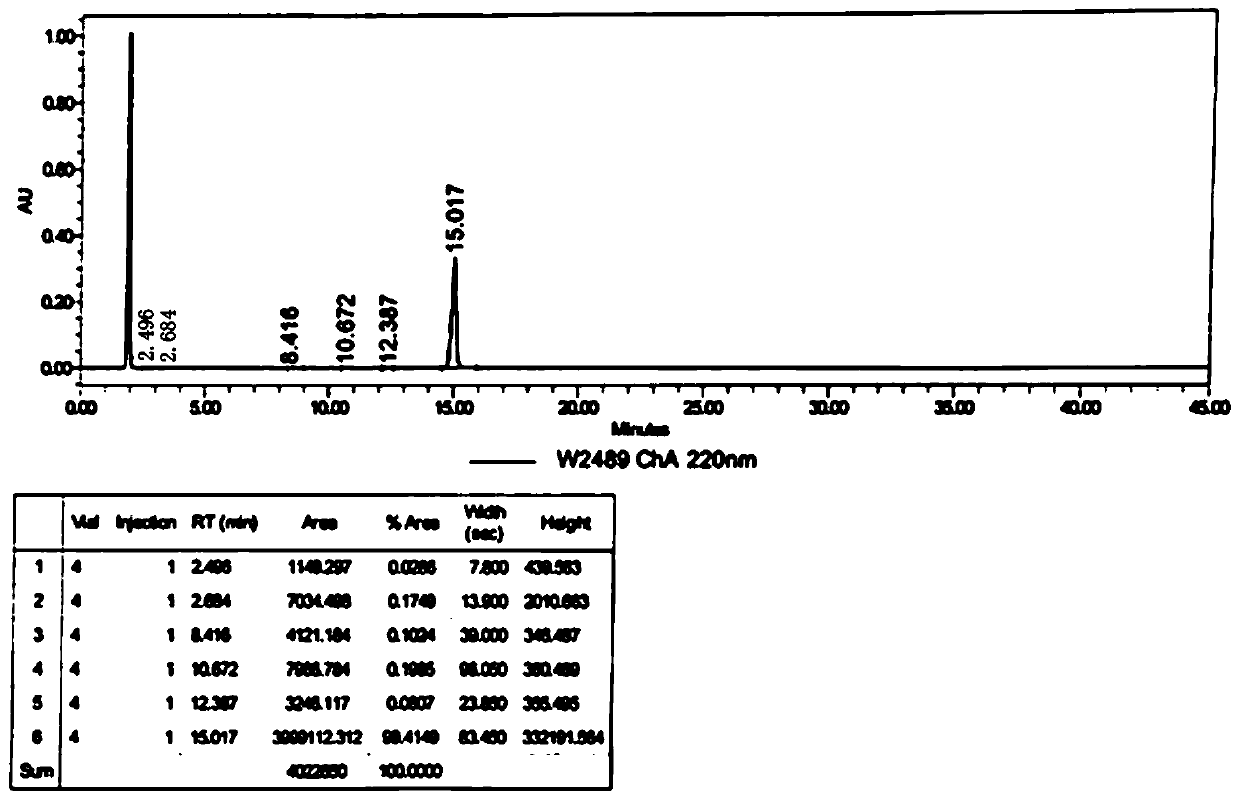
[0054] Embodiment 1, the preparation of semaglutide of the present invention
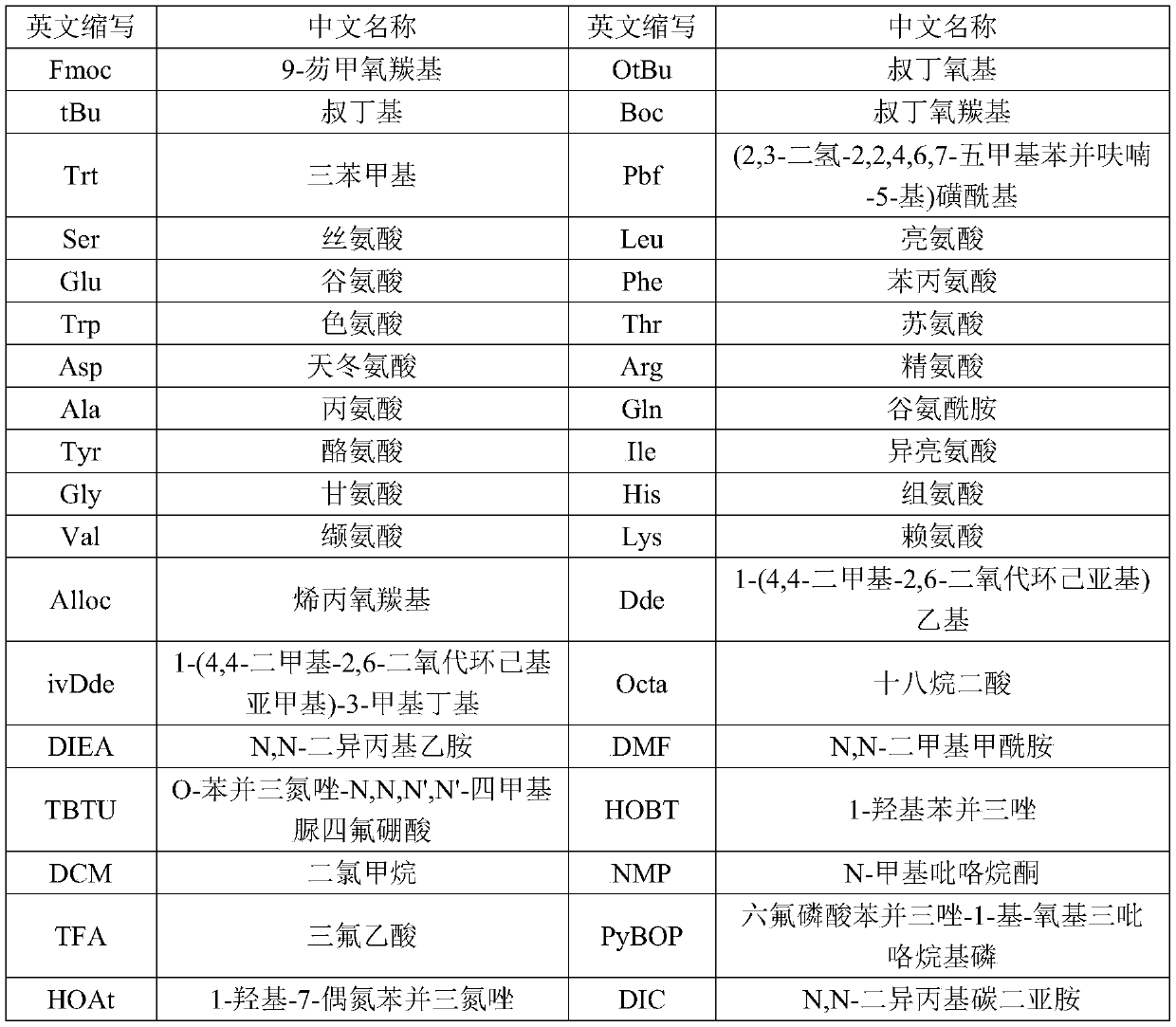
[0055] Using Fmoc-Gly-Wang with a uniform substitution degree of 0.28mmol / g (purchased from Xi'an Lanxiao Technology New Material Co., Ltd., batch number UN104F08-190221) as the initial resin carrier, through de-Fmoc protection and coupling reaction, sequentially combined with the table The protected amino acids shown in 2 were coupled to prepare semaglutide peptide resin.
[0056] Table 2 The protected amino acids used in the present invention
[0057] The peptide sequence n= Protected Amino Acids molecular weight 2 Fmoc-Arg(Pbf)-OH 648 3 Fmoc-Gly-OH 297 4 Fmoc-Arg(Pbf)-OH 648 5 Fmoc-Val-OH 339 6 Fmoc-Leu-OH 353 7 Fmoc-Trp(Boc)-OH 526 8 Fmoc-Ala-OH 311 9 Fmoc-Ile-OH 353 10 Fmoc-Phe-OH 387 11 Fmoc-Glu(OtBu)-OH 425 12 Fmoc-Lys(Alloc)-OH 452 13 Fmoc-Ala-OH 311 14 Fmoc-Ala-OH 311 15 Fmoc-Gln(T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com