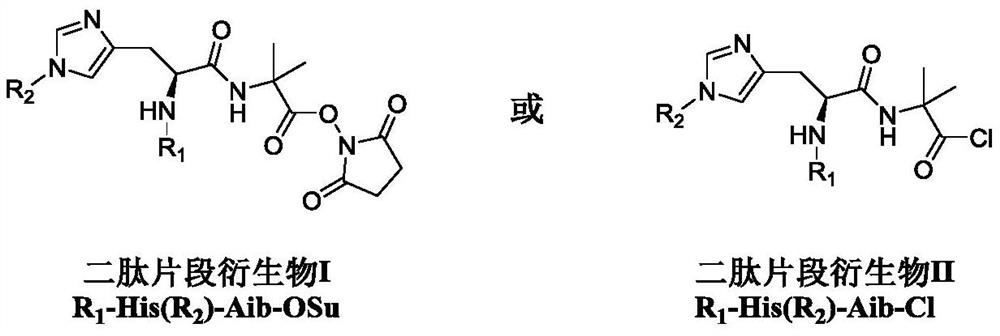
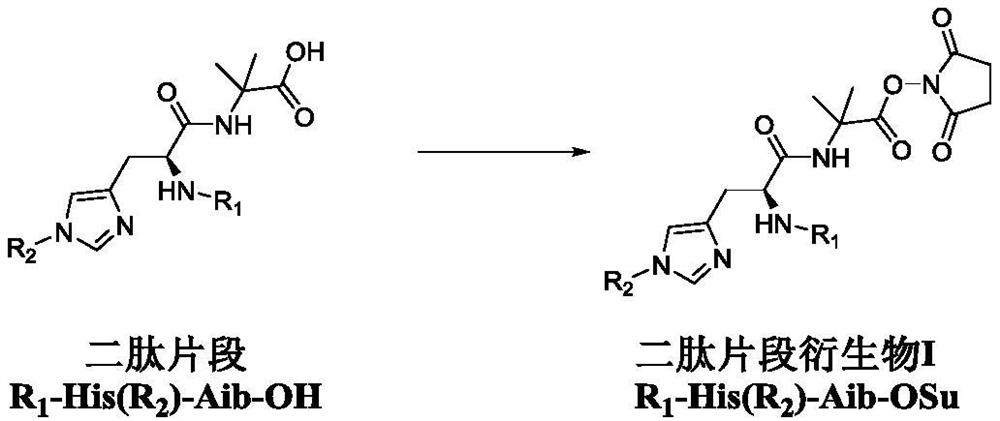
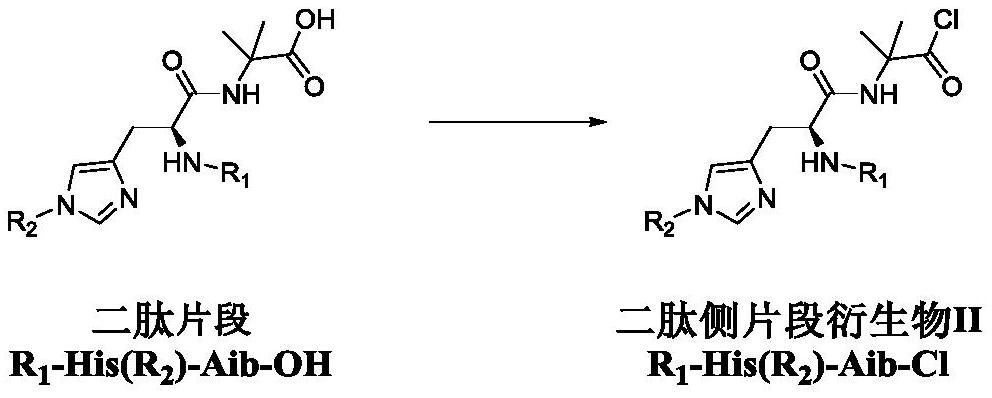
Dipeptide fragment derivative for synthesizing semaglutide and preparation method of dipeptide fragment derivative
A technology for semaglutide and peptide fragments, which is applied in the field of dipeptide fragment derivatives synthesized by semaglutide and its synthesis field, can solve the problems of difficult control of isomer content, low optical purity of products, His racemization and the like , to achieve the effect of improving the coupling efficiency, improving the reaction efficiency, and facilitating the production scale.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Synthesis of Intermediate I
[0045]
[0046] R 1 =Fmoc, R 3 =Trt
[0047] Weigh 50.01g Fmoc-L-His(Trt)-OH into 500ml tetrahydrofuran, stir, add 18.95g DIPEA (2.00eq) to the reaction flask, stir to dissolve, and add 20.12g EDCI (1.30eq) to the reaction flask in turn , 14.21g HOBt (1.30eq), 18.95g 2-aminoisobutyric acid tert-butyl ester hydrochloride (1.20eq), stirred for 5 hours, the reaction was complete, the reaction solution was concentrated, the residue was dissolved in 300ml EA, and the organic phase was washed with 300ml purified water 2 times, wash once with 300ml of saturated sodium chloride aqueous solution, separate the liquid, collect the organic phase, concentrate the organic phase to 100g, control the temperature to 0-15°C, add the concentrated dropwise to 750ml of n-heptane for crystallization, and after the addition is complete , stirred at 0-15°C for 30 minutes, filtered, and the filter cake was vacuum-dried to obtain 65.90 g of white so...
Embodiment 2
[0048] Example 2: Synthesis of Intermediate I
[0049]
[0050] R 1 =Fmoc, R 3 =Fmoc
[0051] Weigh 50.00g Fmoc-L-His(Fmoc)-OH into 500ml dichloromethane, stir, add 21.55g DIPEA (2.00eq) to the reaction flask, stir to dissolve, and add 14.21g DIC (1.35g) to the reaction flask in turn eq), 15.21g HOBt (1.35eq), 19.58g 2-aminoisobutyric acid tert-butyl ester hydrochloride (1.20eq), stirred for 6 hours, the reaction was complete, the reaction solution was concentrated, the residue was dissolved in 300ml EA, and the organic phase was dissolved in 300ml Washed twice with purified water, washed once with 300ml of saturated aqueous sodium chloride solution, separated, collected the organic phase, concentrated the organic phase to 100g, controlled the temperature to 0-10°C, added the concentrated dropwise to 750ml of petroleum ether for crystallization, and finished the dropwise addition After that, the mixture was kept at 0-10°C and stirred for 30 minutes, filtered, and the fil...
Embodiment 3
[0052] Example 3: R 1 -His(R 2 Synthesis of )-Aib-OH
[0053]
[0054] R 1 =Fmoc, R 3 =Trt or Mmt or Mtt, R 2 =H
[0055] At room temperature, weigh 62.66g of intermediate I crude product and add it to 250ml of dichloromethane, stir and dissolve, measure 188ml of trifluoroacetic acid and add it to the reaction flask, add 25.90g of triisopropylsilane, stir at room temperature for 4 hours, and the raw materials react. Complete, the reaction solution was concentrated, the concentration was completed, the residue 1378ml isopropyl ether was slurried at room temperature, filtered, 564ml of acetonitrile was added to the filter cake, slurried at room temperature, and dried to obtain 36.13g white solid powder terminal dipeptide fragment, purity 98.27%, yield 70.50 %, the isomer content is 0.05%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com