Continuous flow solid-phase reaction preparation of semaglutide
A technology of fluid-solid and condensation reaction, which is applied in the field of preparation of semaglutide combined with continuous flow solid-phase synthesis system, which can solve the problems of low total yield, low efficiency and good solubility of crude products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
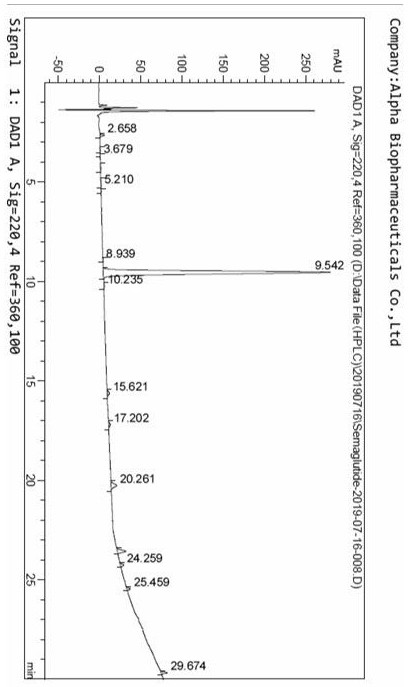
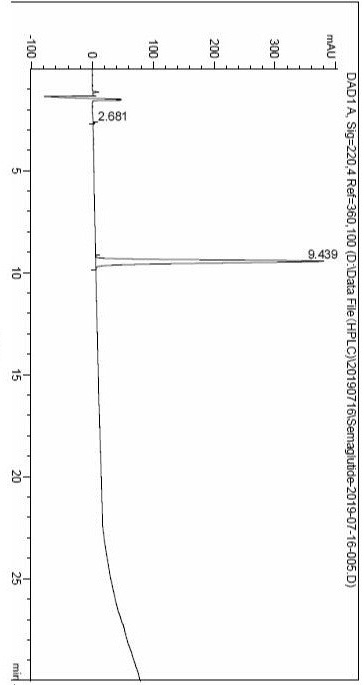
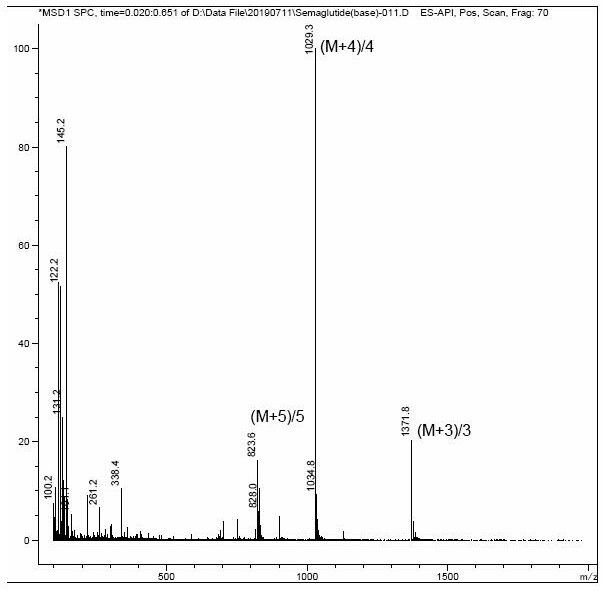
Image
Examples
Embodiment 1
[0107] Step 1: Preparation of Fmoc-Thr(tBu)-Phe-Thr(tBu)-Ser(tBu)-Asp(OtBu)-Val-Ser(Psi(me.me)Pro)-OH Fragment 1
[0108]
[0109] 1) Fmoc-Val-Ser(Psi(me.me)Pro)-2-CTC-resin
[0110] 10g of 2-CTC-resin (substitution degree=0.72mmol / g) was added to the solid phase reactor, the resin was swollen with 120mL of dichloromethane for 5 minutes, and then Fmoc-Val-Ser(Psi(me.me)Pro) was added sequentially -OH 1g (2.16mmol), 10.2mL DIEA, stirred for 2 hours. Drain the solvent, add 100mL of 10:90 methanol / dichloromethane solution, stir for half an hour to block unreacted complete chloromethyl. Wash 3 times with 100 mL of dichloromethane each time. After vacuum drying, the substitution degree of Fmoc-Val-Ser(Psi(me.me)Pro)-2-CTC-resin was measured to be 0.61 mmol / g.
[0111] Fmoc-Val-Ser(Psi(me.me)Pro)-2-CTC-ChemMatrix resin was removed with 100mL 2%DBU / 3%1-octylthiol DMF solution for 12 minutes at room temperature, and H- Val-Ser(Psi(me.me)Pro)-2-CTC-resin.
[0112] 2) Fmoc-Thr(t...
Embodiment 2
[0146] Step 1: Fmoc-Gly-HMPA-ChemMatrix resin synthesis
[0147]
[0148] Weigh 15g of HMPA-ChemMatrix resin (degree of substitution = 0.53mmol / g) and add it to a solid-phase reactor, swell the resin with 100mL of dichloromethane for 5 minutes, then add 6.7g of Fmoc-Gly-OH, 8.2mL of DIEA, and stir for 2 hours . Drain the solvent, add 100mL of 10:90 methanol / dichloromethane solution, stir for half an hour to block unreacted complete chloromethyl. Wash 3 times with 100 mL of dichloromethane each time. After vacuum drying, the degree of substitution of the Fmoc-Gly-HMPA-ChemMatrix resin was measured to be 0.47 mmol / g.
[0149] Step 2: H-Ser(tBu)-Tyr(tBu)-Leu-Glu(OtBu)-Gly-Gln(Trt)-Ala-Ala-Lys(AEEA-AEEA-(γ-Glu-(OtBu))-monoButyl Octadecanate)-Glu(OtBu)-Phe-Ile-Ala-Trp(Boc)-Leu-Val-Arg(pbf)-Gly-Arg(pbf)-Gly-HMPA-ChemMatrix resin 3 synthesis
[0150] The Fmoc-Gly-HMPA-ChemMatrix resin synthesized in step 1 was used for semaglutide resin synthesis. Weigh 80 g of Fmoc-Gly-HMPA-...
Embodiment 3
[0166] Step 1: Fmoc-Gly-Trt-ThioPEG-AM-PS resin synthesis
[0167]
[0168] Weigh 15g of Cl-Trt-ThioPEG-AM-PS resin (degree of substitution = 0.39mmol / g) and add it to a solid-phase reactor, swell the resin with 100mL of dichloromethane for 5 minutes, then add 5.2g of Fmoc-Gly-OH in sequence, 7.4 mL DIEA, stirred for 3 hours. Drain the solvent, add 100mL of 10:90 methanol / dichloromethane solution, and stir for half an hour to block unreacted triphenylchloromethyl. Wash 3 times with 100 mL of dichloromethane each time. After vacuum drying, the degree of substitution of the Fmoc-Gly-Trt-ThioPEG-AM-PS resin was measured to be 0.32 mmol / g.
[0169] Step 2: H-Ser(tBu)-Tyr(tBu)-Leu-Glu(OtBu)-Gly-Gln(Trt)-Ala-Ala-Lys(AEEA-AEEA-(γ-Glu-(OtBu))-monoButyl Octadecanate)-Glu(OtBu)-Phe-Ile-Ala-Trp(Boc)-Leu-Val-Arg(Boc) 2 -Gly-Arg(Boc) 2 -Gly-Trt-ThioPEG-AM-PS resin 3 synthesis
[0170] The Fmoc-Gly-Trt-ThioPEG-AM-PS resin synthesized in step 1 was used for semaglutide resin synthes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com