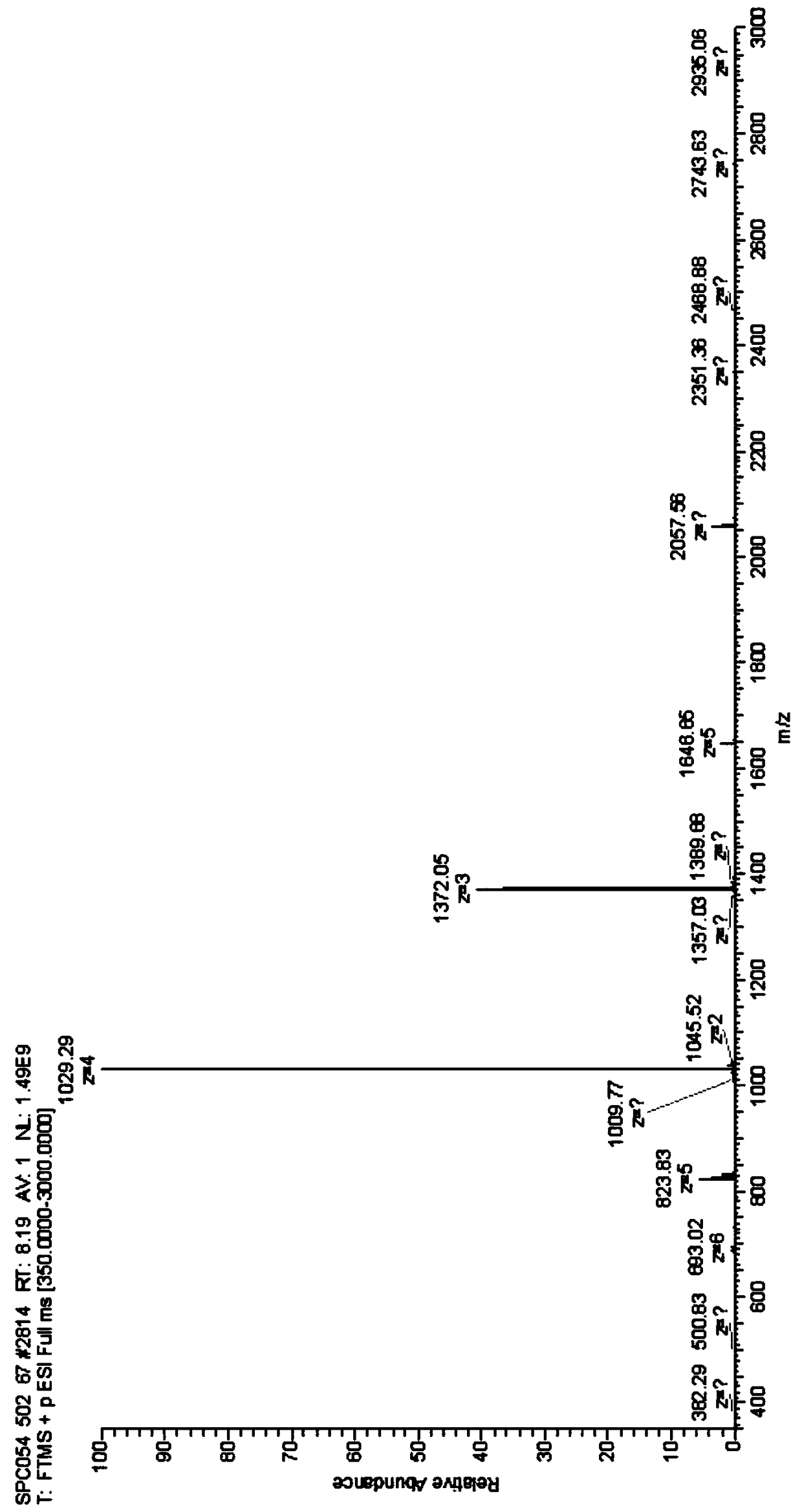
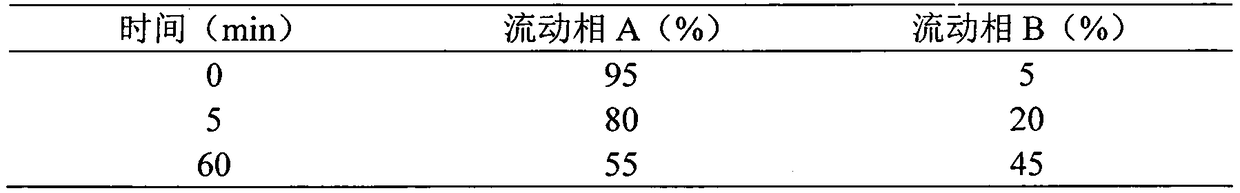
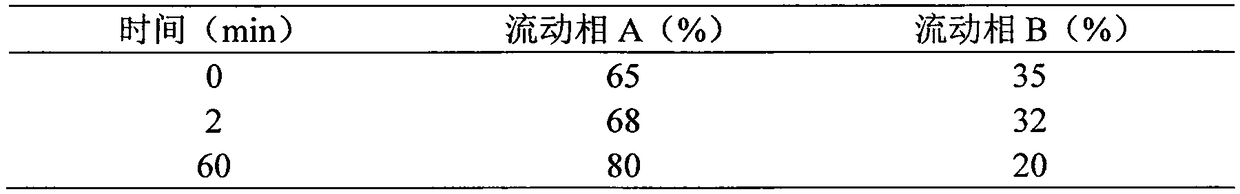
Synthetic method for semaglutide
A technology of semaglutide and a synthesis method, which is applied in the field of polypeptide drug synthesis, can solve the problems of destroying the secondary structure of straight-chain peptides, unfavorable to industrialized production, low purity and yield, etc., so as to reduce costs and solve the problem of difficult coupling. , synthesizing simple and easy-to-operate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0047] The technical solutions of the present invention are further described below, so that those skilled in the art can further understand the present invention, without constituting a limitation on the rights of the present invention.
[0048] One, the preparation of Fmoc-Gly-Wang resin
[0049] Weigh 110.0 g of Wang resin (Sub=0.61 mmol / g) and add it to a solid-phase reactor, add 360 mL of DCM to swell the resin for 0.5 h, and drain the solvent. Add 49.1g of Fmoc-Gly-OH and 29.5g of HOBt to 360ml of DMF solution and stir to dissolve. After cooling down to 5-8 degrees, add 29.5mL of DIC solution and stir. Add the activated solution to the resin and stir for 5h. Drain the solvent, add 11 mL of acetic anhydride, 8 mL of pyridine, and 170 mL of DMF, and block for 2 hours. DCM and methanol were alternately washed 3 times, 170 mL each time. After vacuum drying, the substitution degree of Fmoc-Gly-Wang resin is about Sub=0.55mmol / g.
[0050] 2. Fmoc-Val 10 -Ser 11 Preparatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com