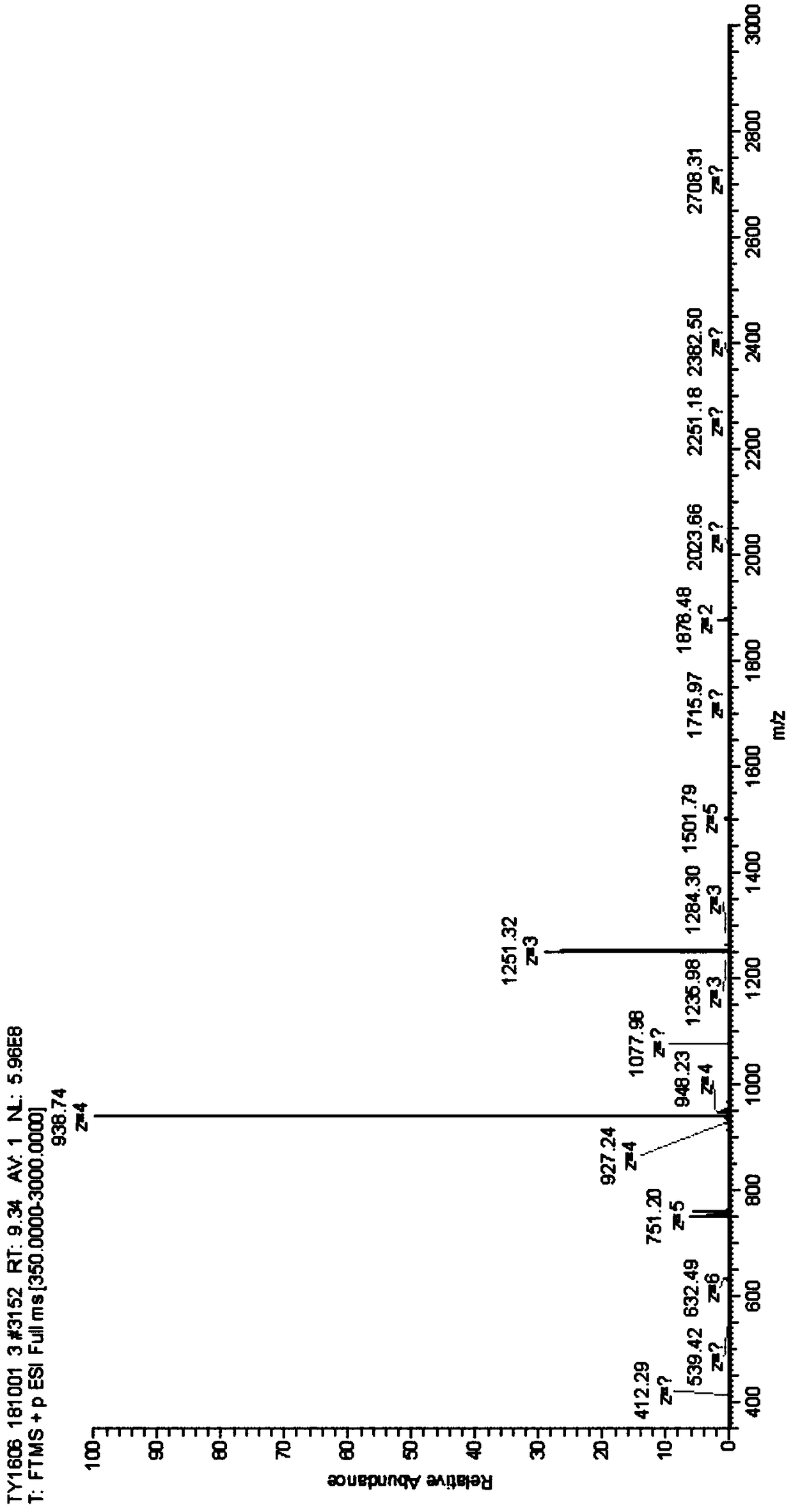
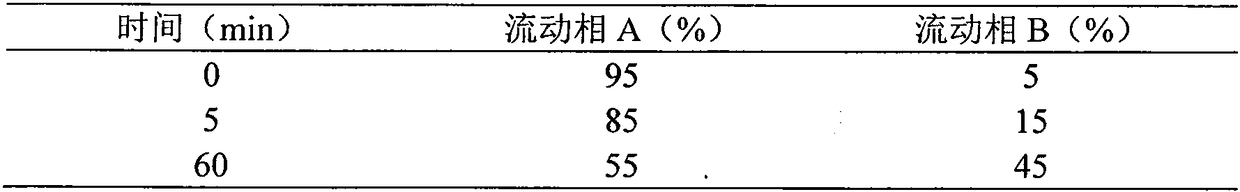
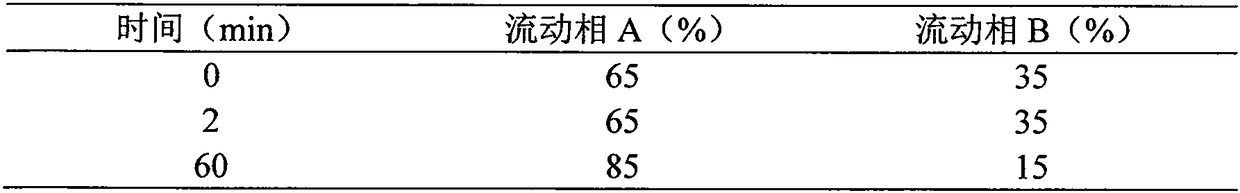
Synthesis method of liraglutide
A technology of liraglutide and synthetic methods, which is applied in the field of polypeptide drug synthesis, can solve the problems of unfavorable industrial production, cumbersome operation steps, low purity and yield, etc., to solve the difficult problem of coupling, reduce costs, and increase yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0045] The technical solution of the method of the present invention is further described below, so that those skilled in the art can further understand the present invention, without constituting a limitation on the claims of the present invention.
[0046] One, the preparation of Fmoc-Gly-Wang resin
[0047] Weigh 100.0 g of Wang resin (Sub=0.61 mmol / g) and add it to a solid-phase reactor, add 350 mL of DCM to swell the resin for 0.5 h, and drain the solvent. Add 45.1g of Fmoc-Gly-OH and 30.5g of HOBt to 370ml of DMF solution and stir to dissolve. After cooling down to 5-8 degrees, add 30.5mL of DIC solution and stir. Add the activated solution to the resin and stir for 5h. Drain the solvent, add 11 mL of acetic anhydride, 8 mL of pyridine, and 160 mL of DMF, and block for 2 hours. DCM and methanol were alternately washed 3 times, 170 mL each time. After vacuum drying, the substitution degree of Fmoc-Gly-Wang resin is about Sub=0.55mmol / g.
[0048] 2. Fmoc-Val 10 -Ser 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com