Synthesizing method of sermaglutide
A technology of semaglutide and synthesis method, which is applied in the field of peptide synthesis, can solve problems such as the synthesis of difficult sequences of semaglutide, and achieve the effects of solving coupling difficulties, reducing costs, and reducing material input
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0059] The present invention will be specifically introduced below in conjunction with the accompanying drawings and specific embodiments.
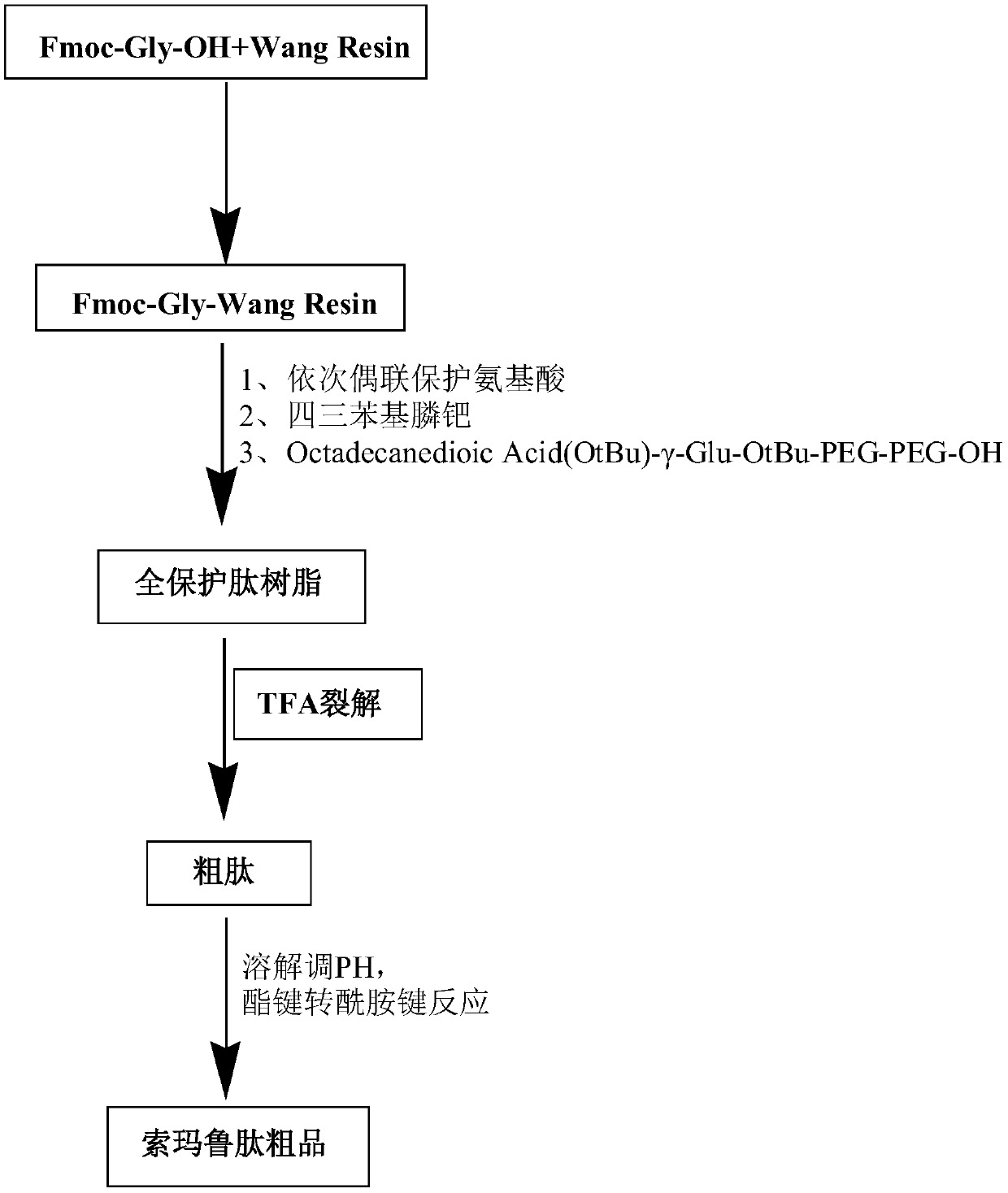
[0060] There are two options for preparing semaglutide:
[0061] Such as figure 1 Option 1 shown;
[0062] A synthetic method for semaglutide, comprising the steps of:
[0063] Step 1, prepare Fmoc-Gly-Wang resin;
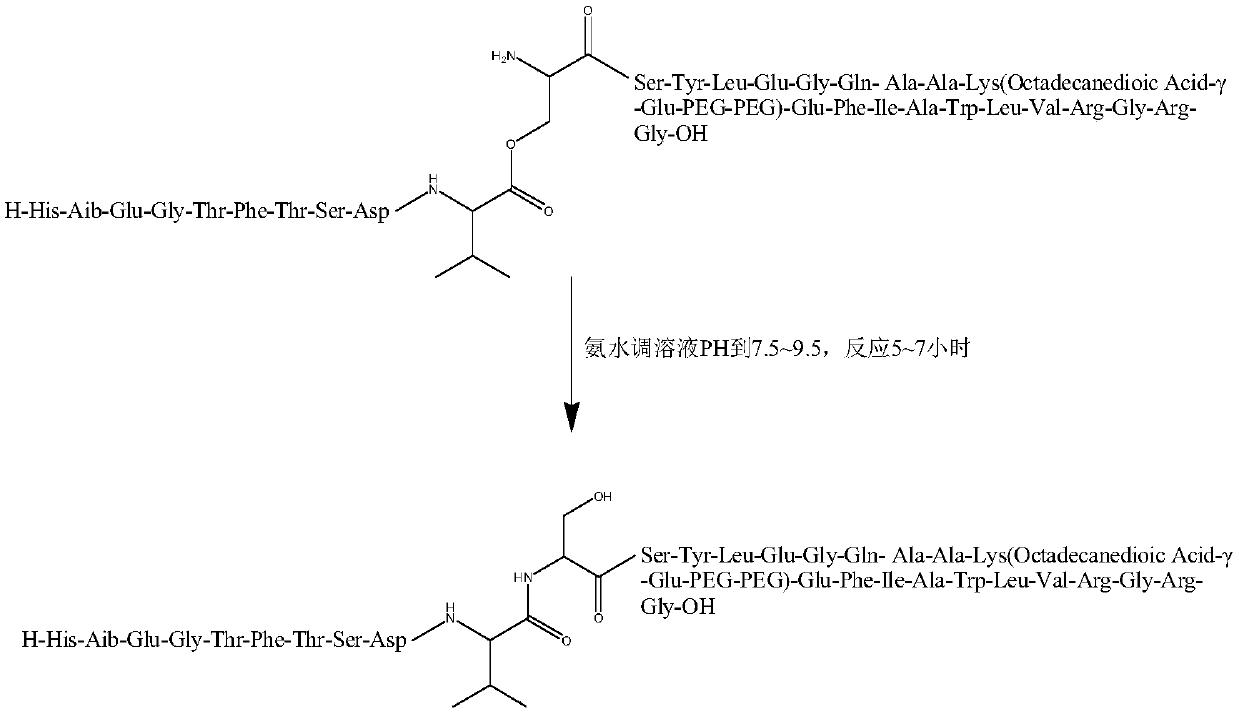
[0064] Step 2, preparing fully protected peptide resin;
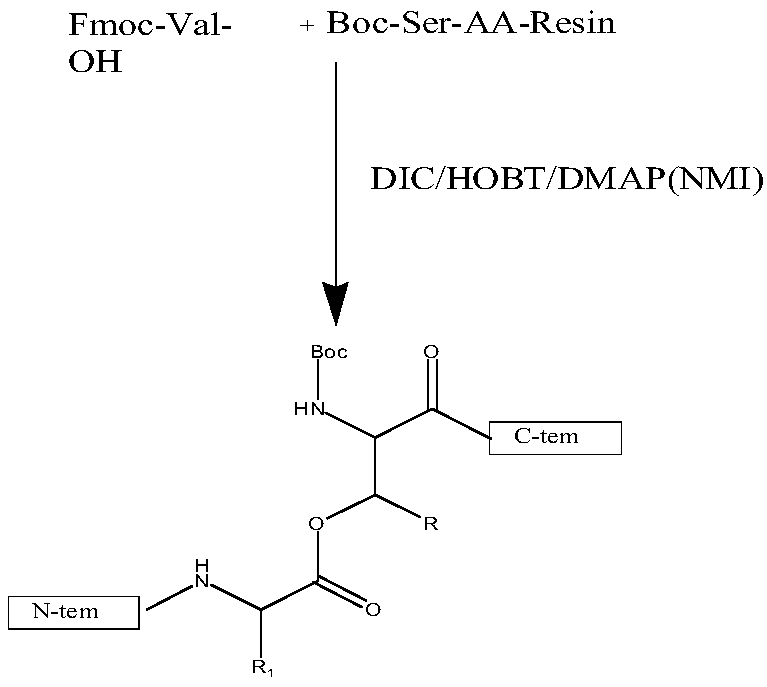
[0065] Step a, preparing protected amino acid fragments,
[0066] The chemical structural formula of the protected amino acid fragment is:
[0067] Where R includes: H, CH 3 (Ser,Thr); Thr in peptide sequence 5 、Thr 7 、Ser 8 、Ser 11 、Ser 12 .
[0068] Written as a chemical formula: Boc-Thr 5 (Fmoc-Gly 4 )-OH, Boc-Thr 7 (Fmoc-Phe 6 )-OH, Boc-Ser8(Fmoc-Thr 7 (tBu))-OH, Boc-Ser 11 (Fmoc-Val 10 )-OH, Boc-Ser 12 (Fmoc-Ser 11 (tBu))-OH.
[0069] Step b, Fmoc-Gly-Wang resin is added in the solid phase reactor;
[0070] Step c, adding the single protect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com