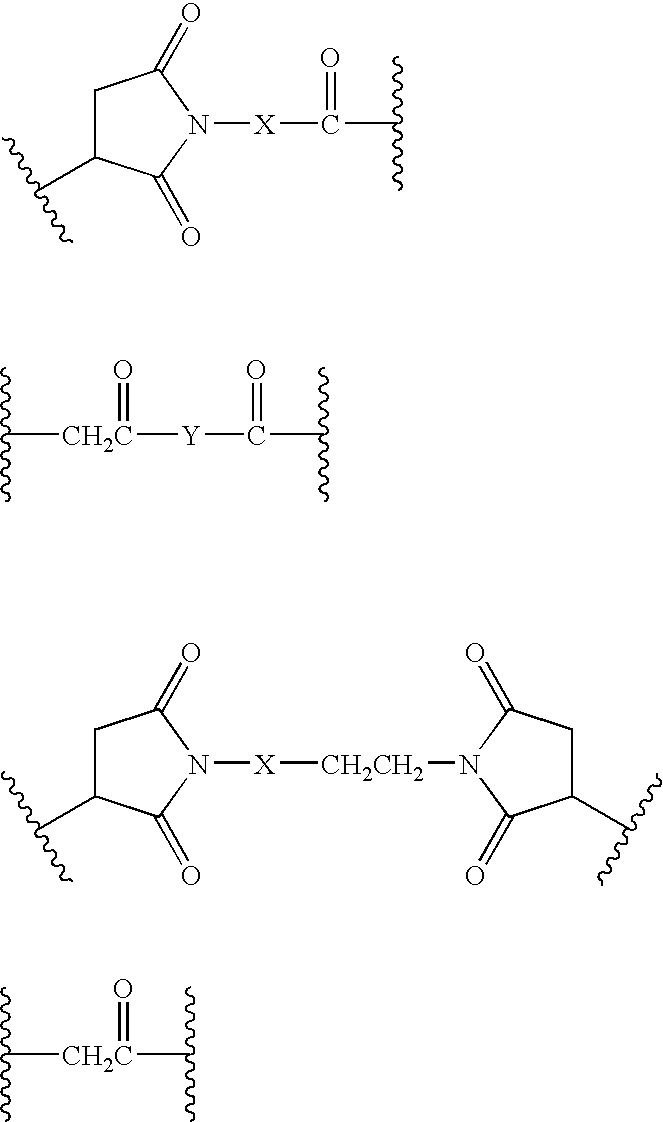
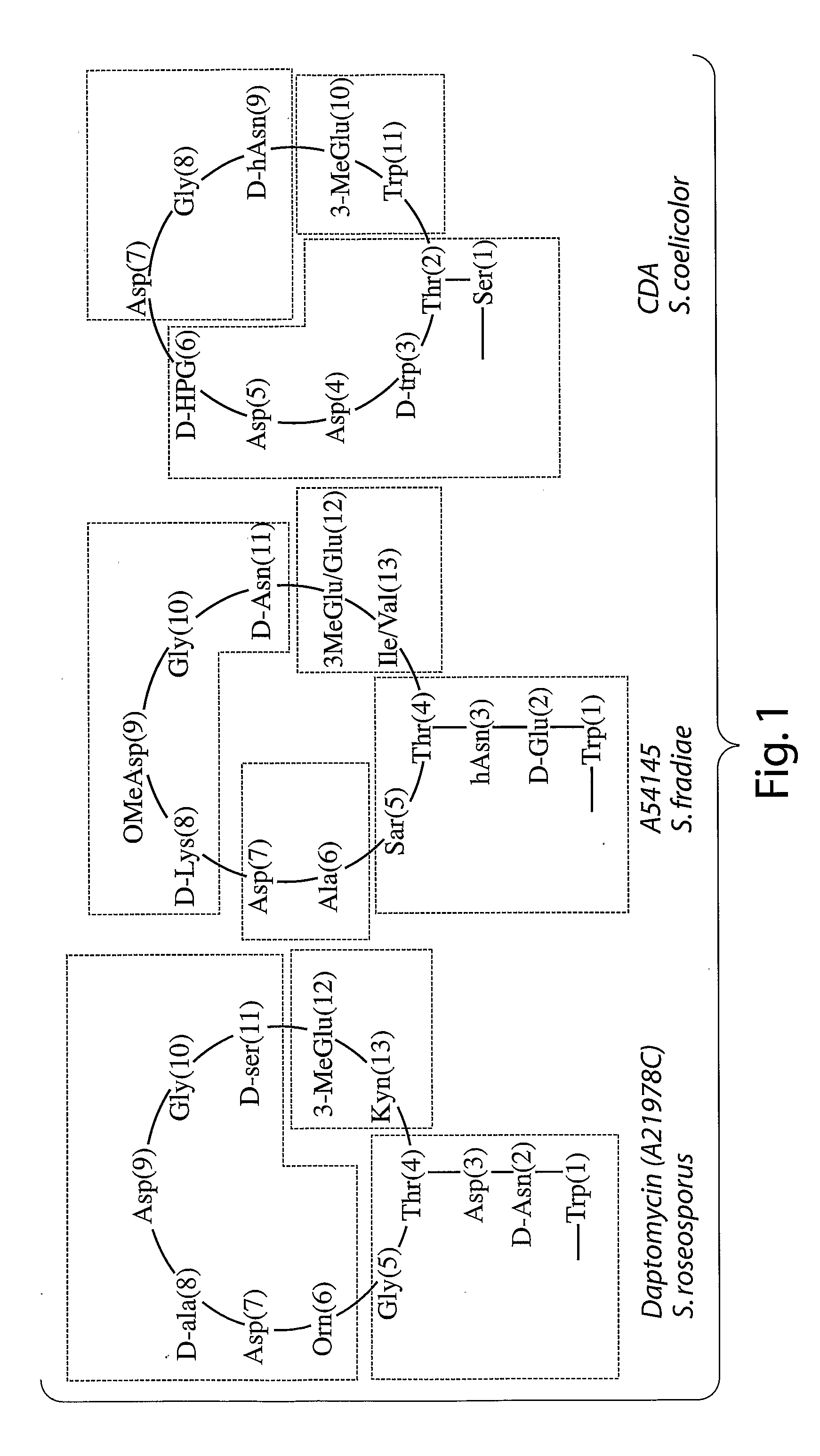
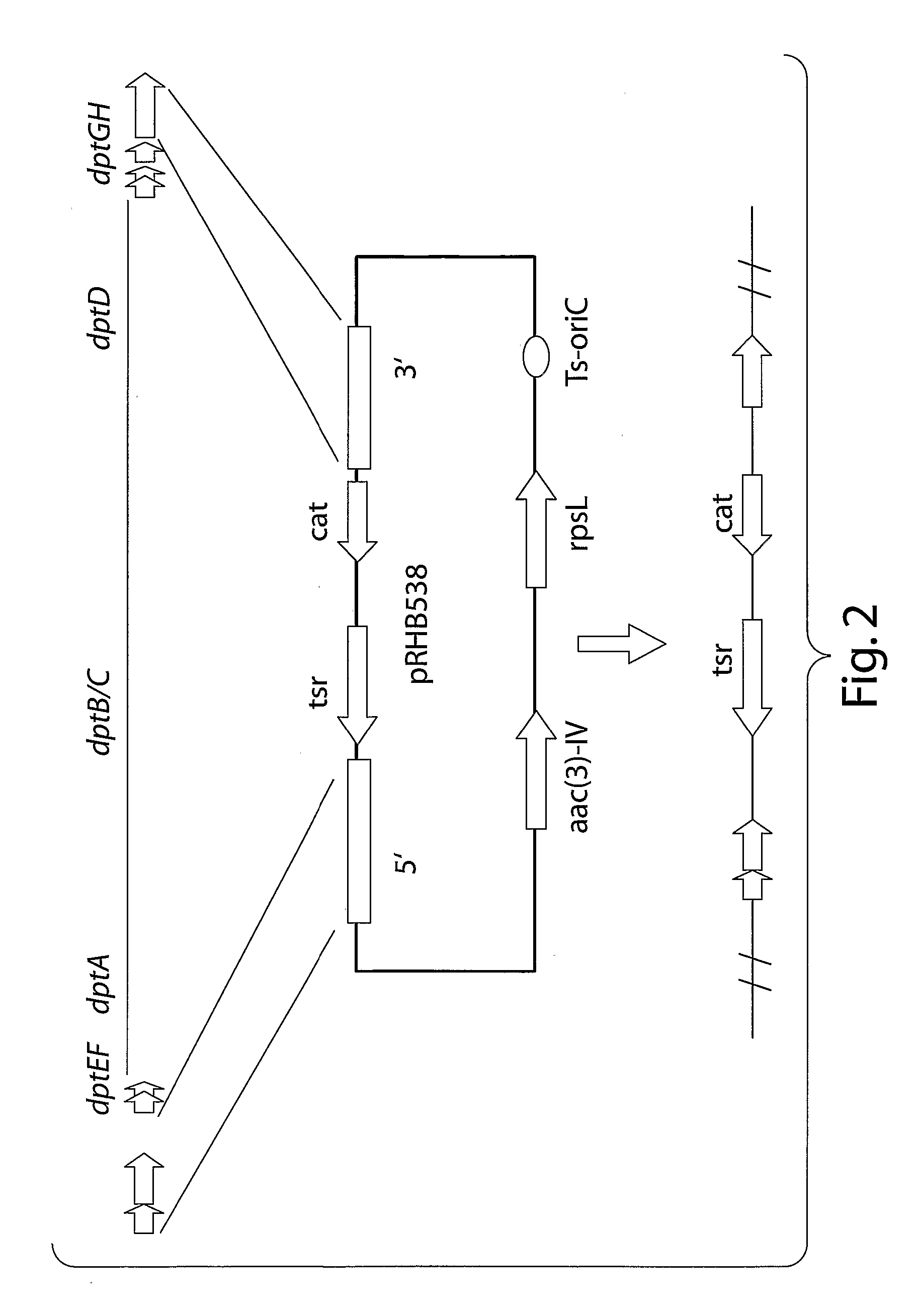
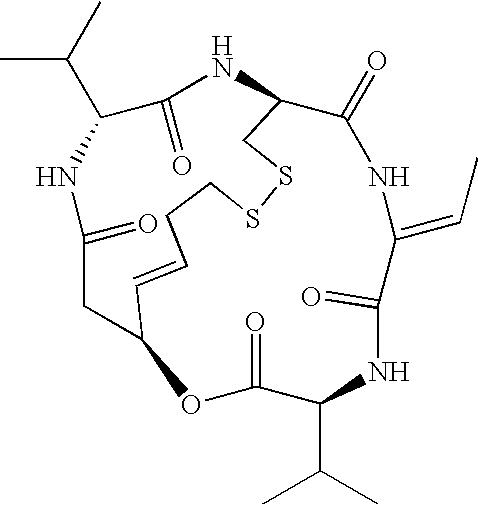
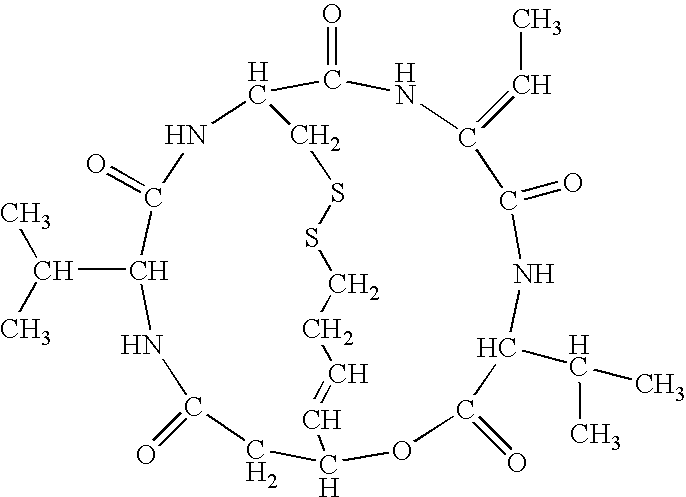
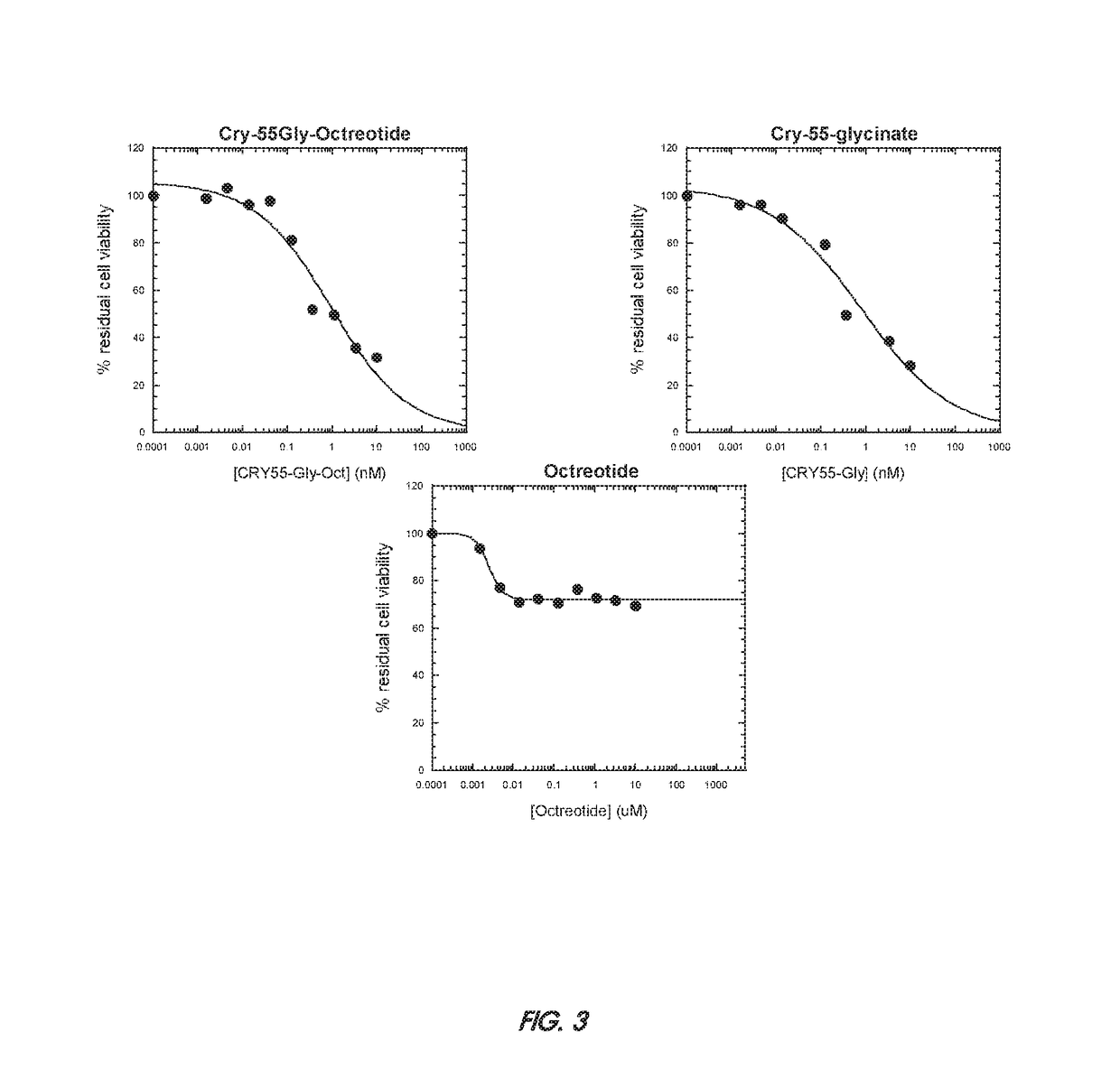
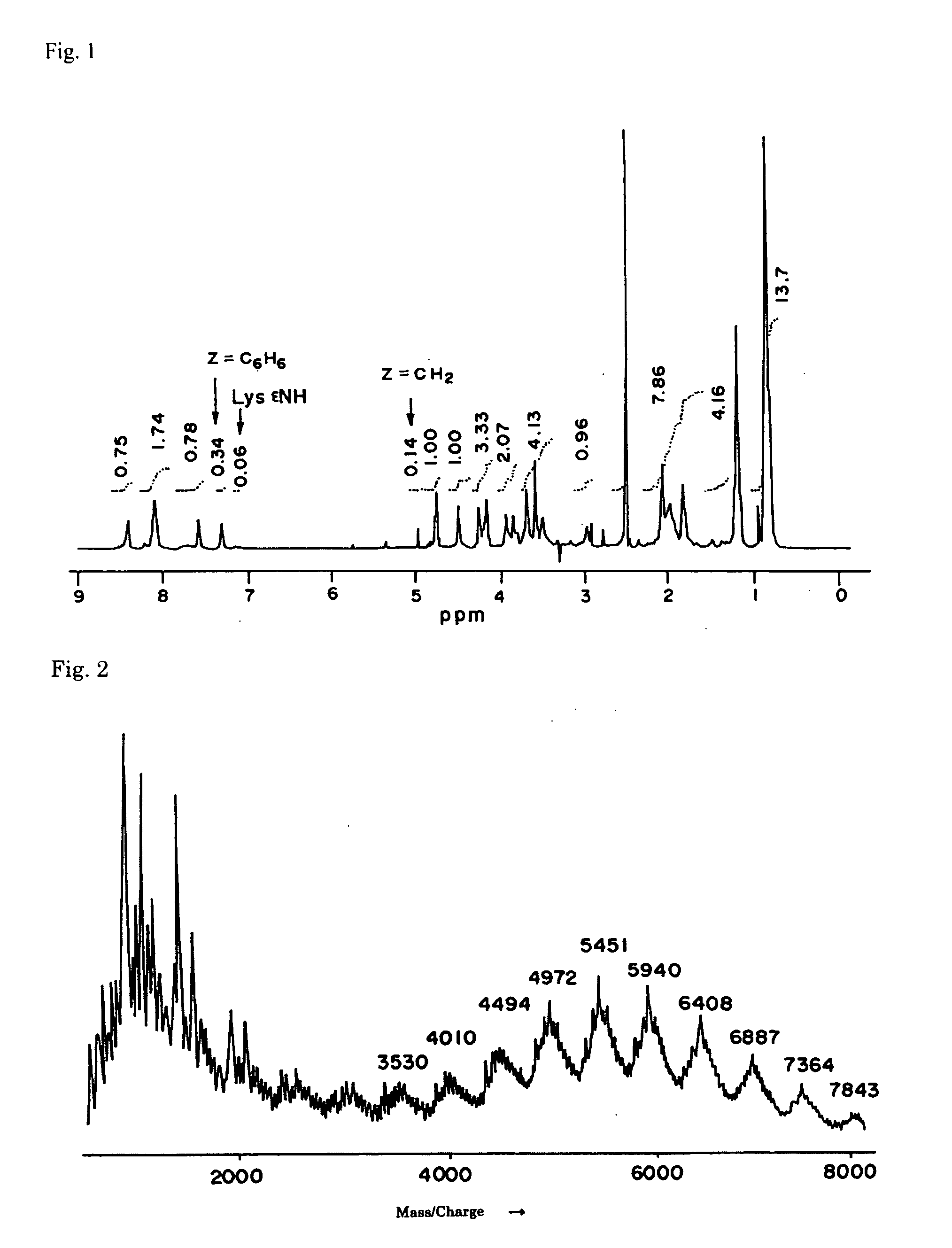
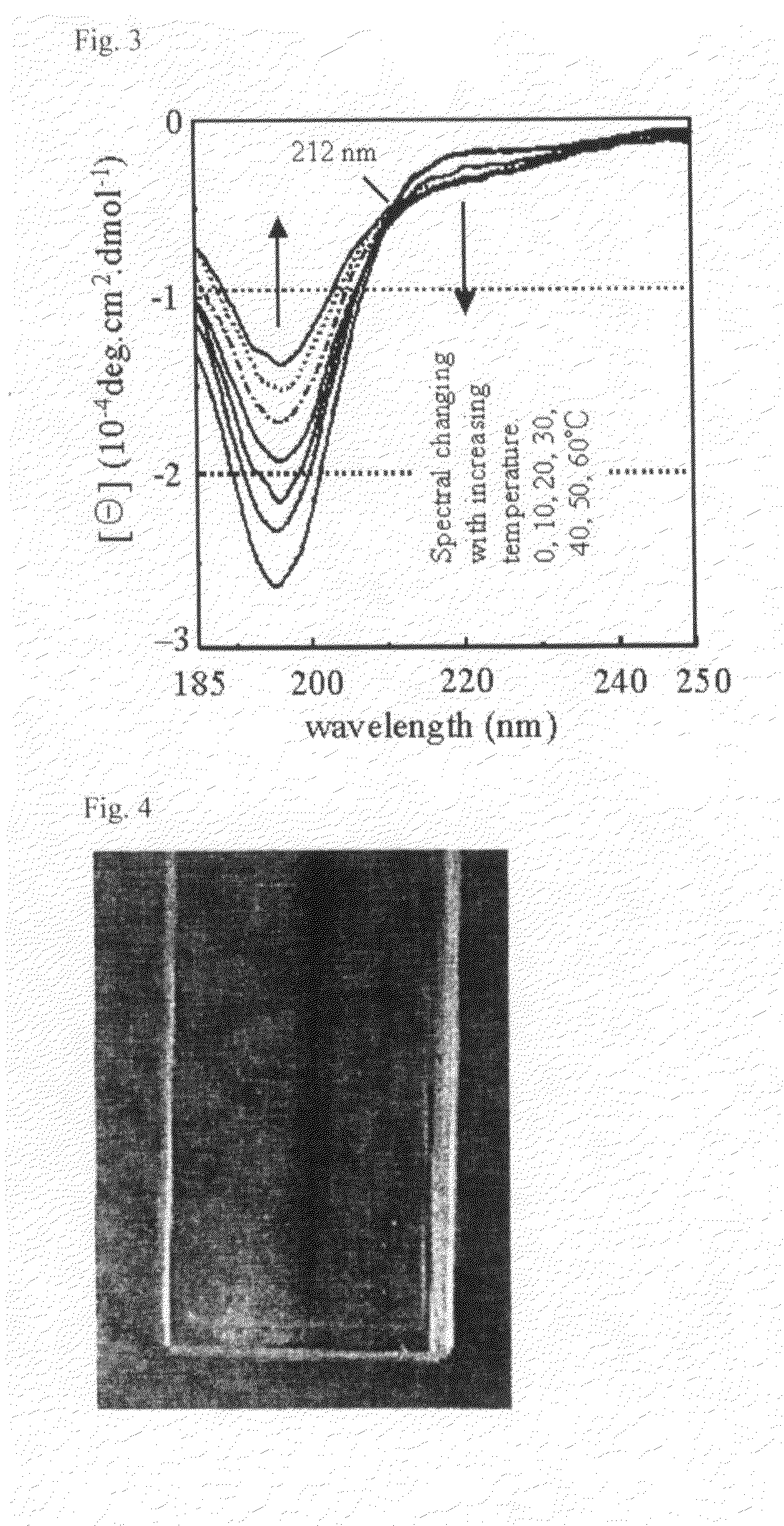
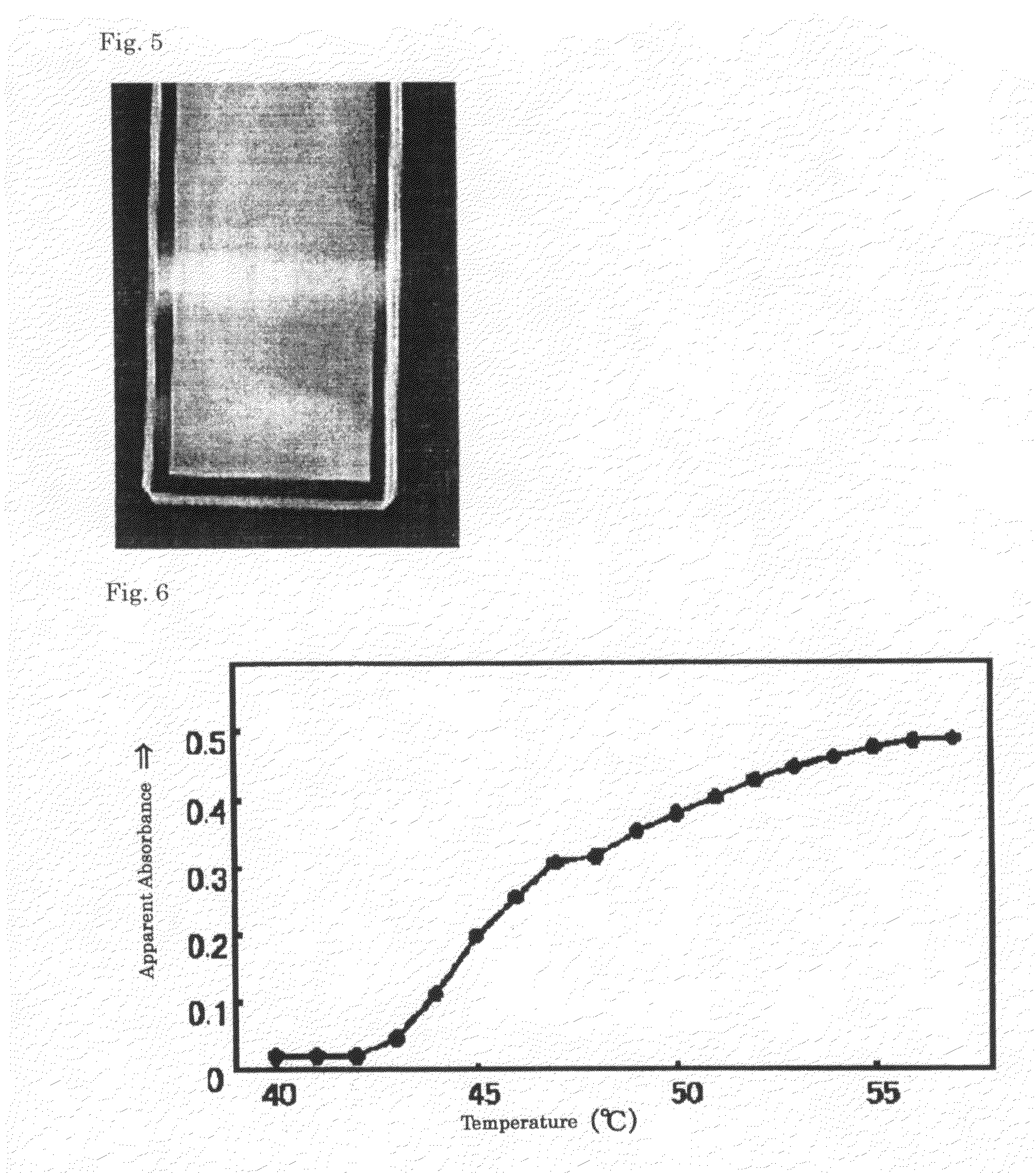
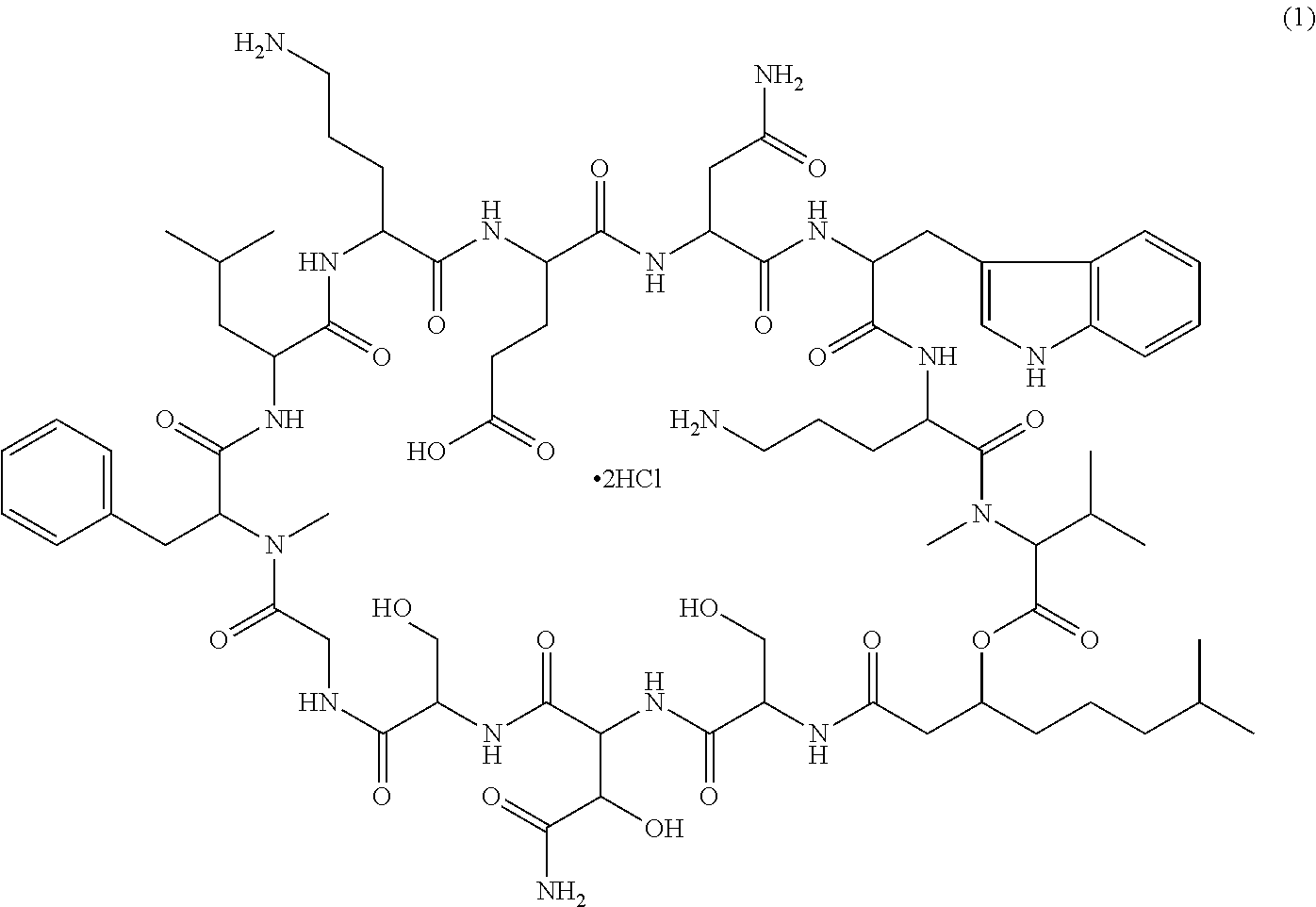
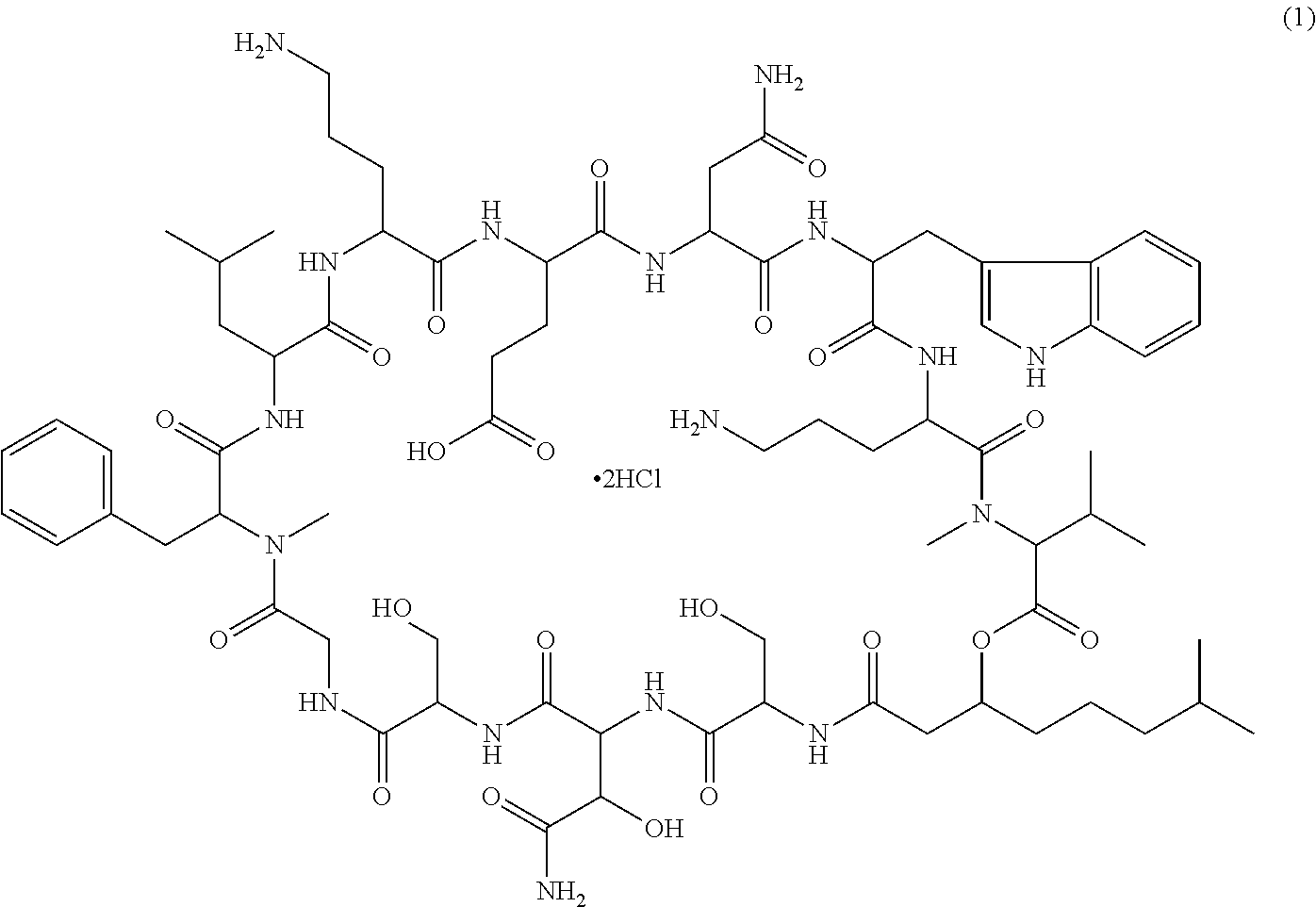
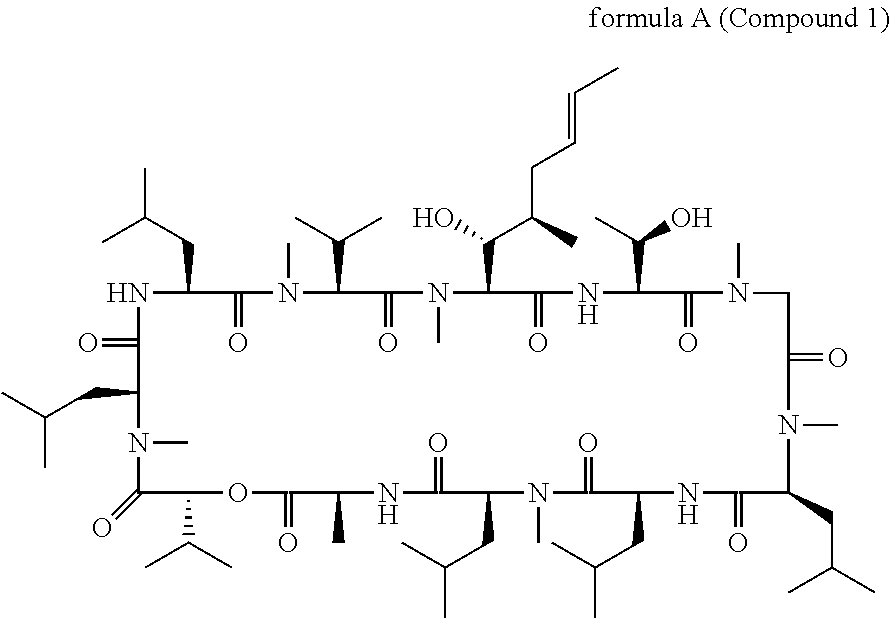
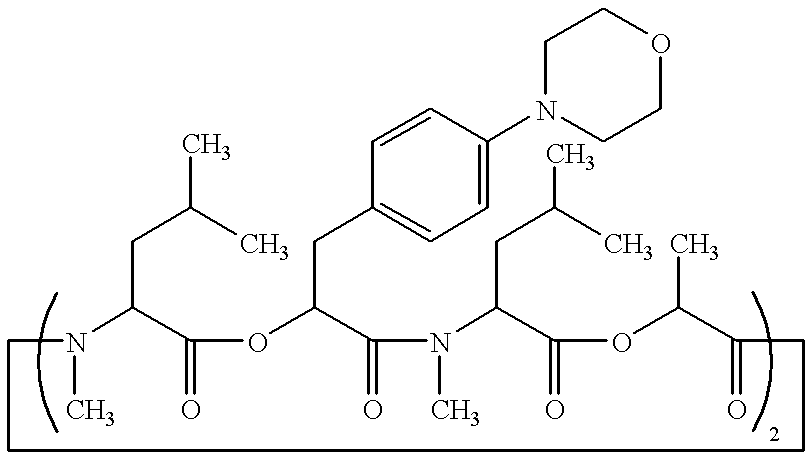
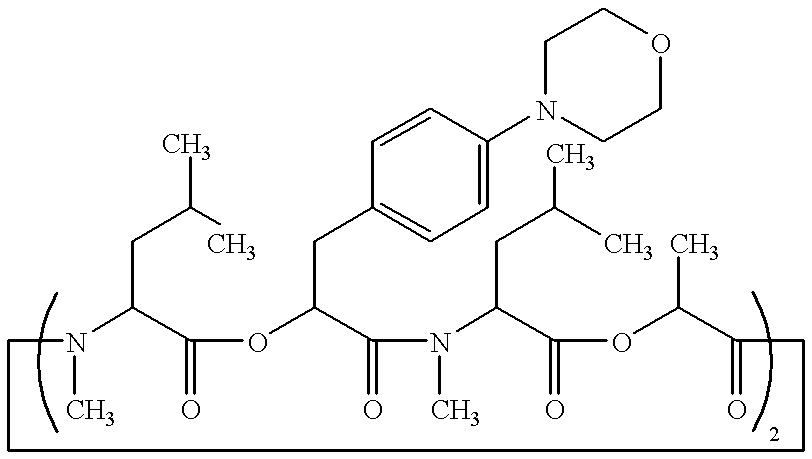
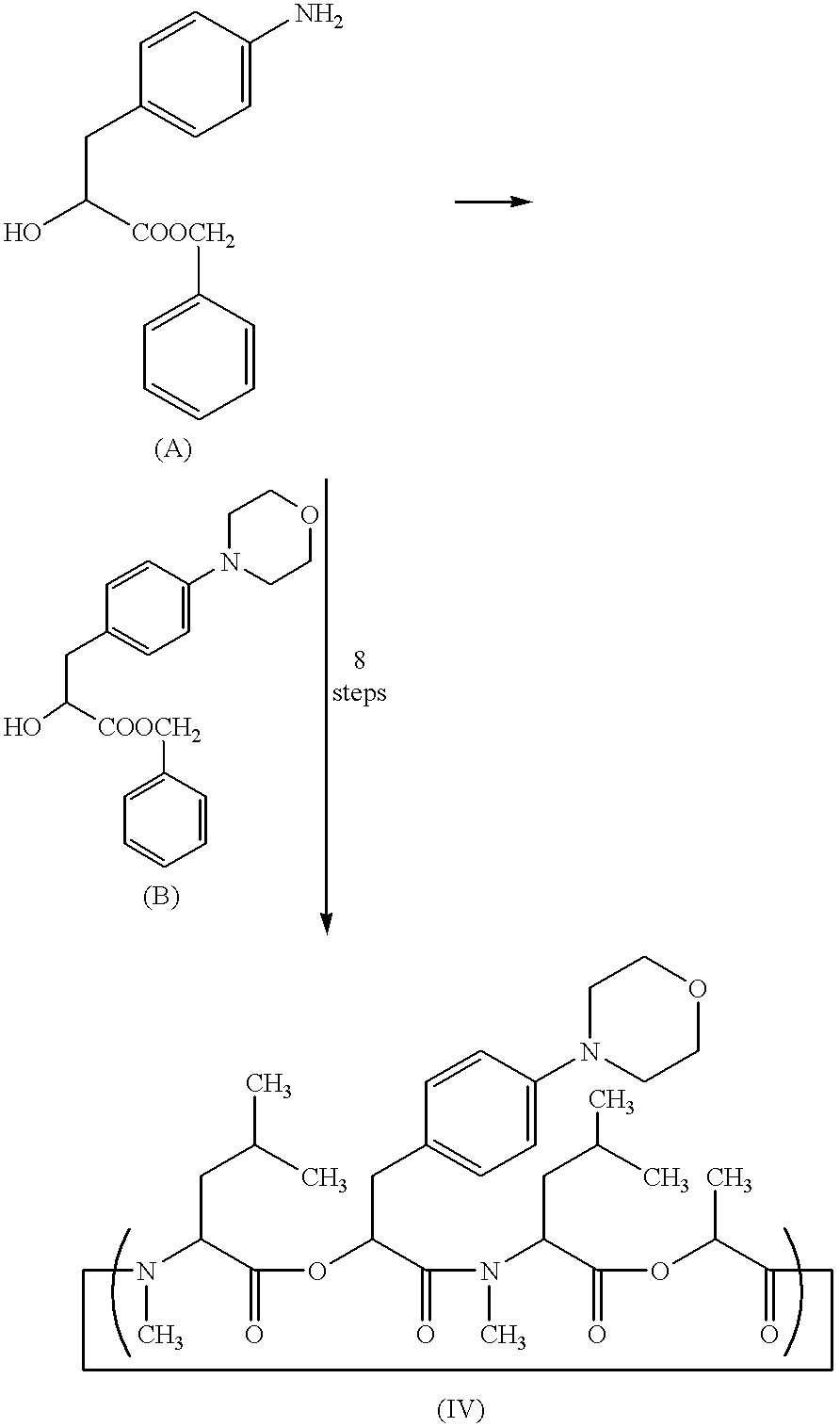
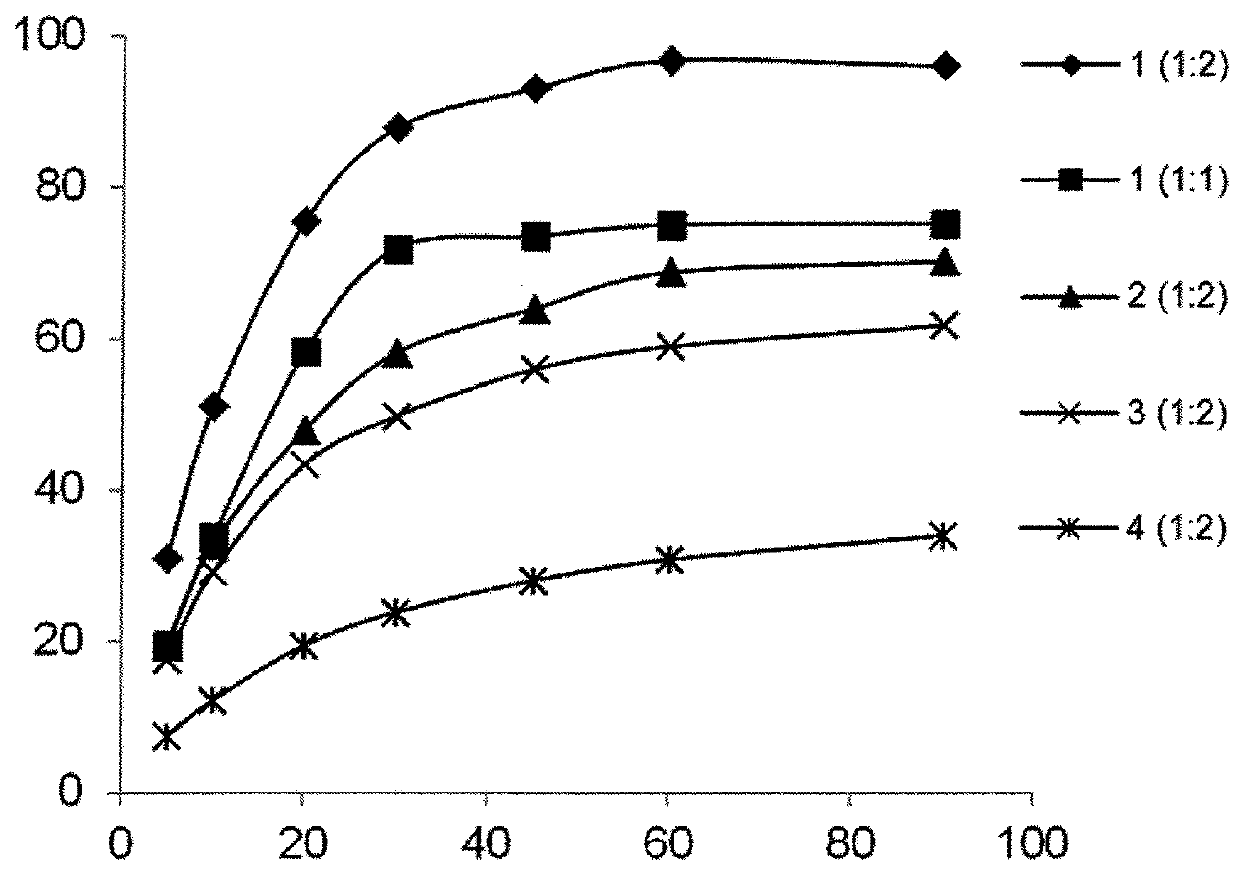
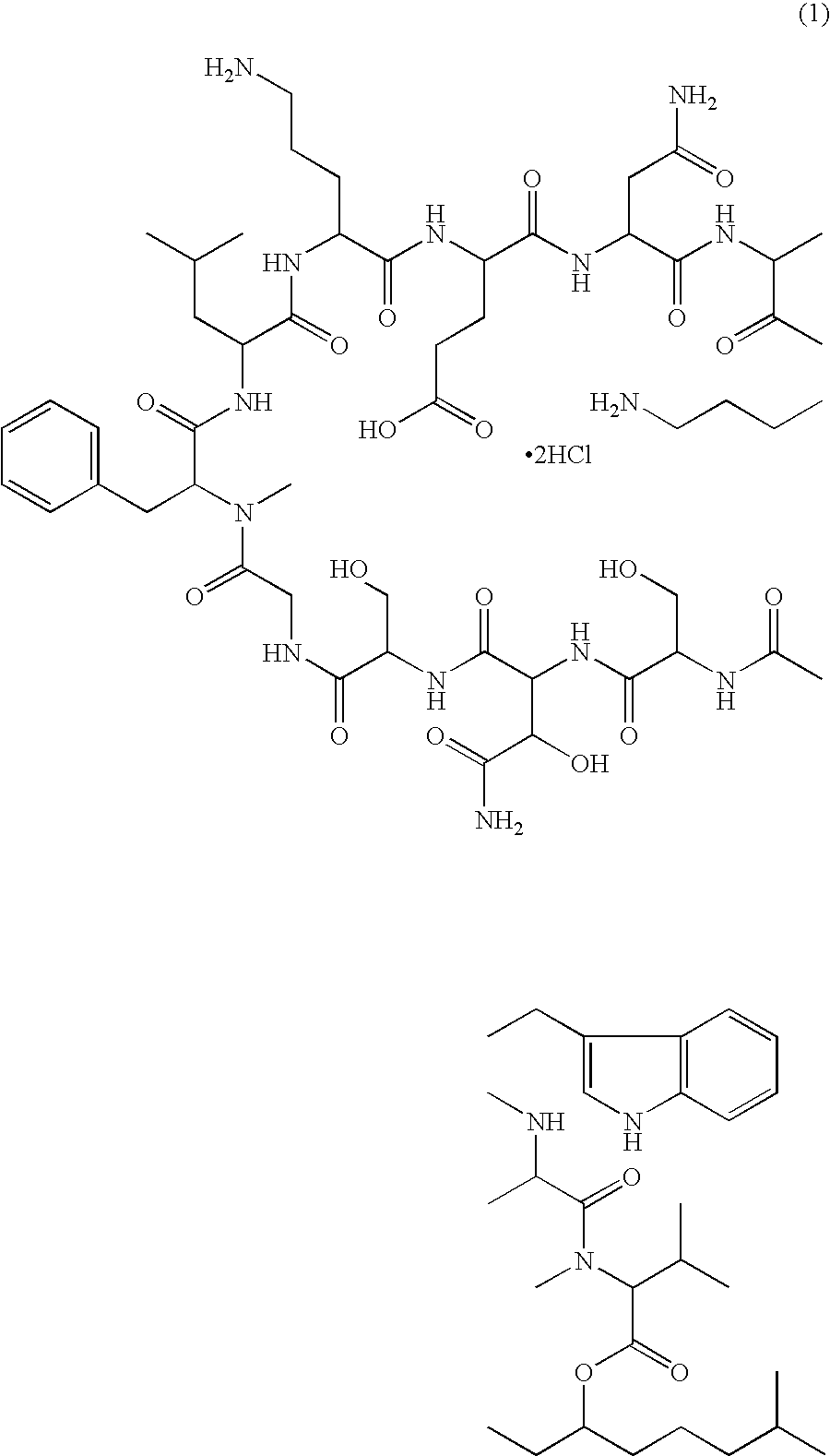
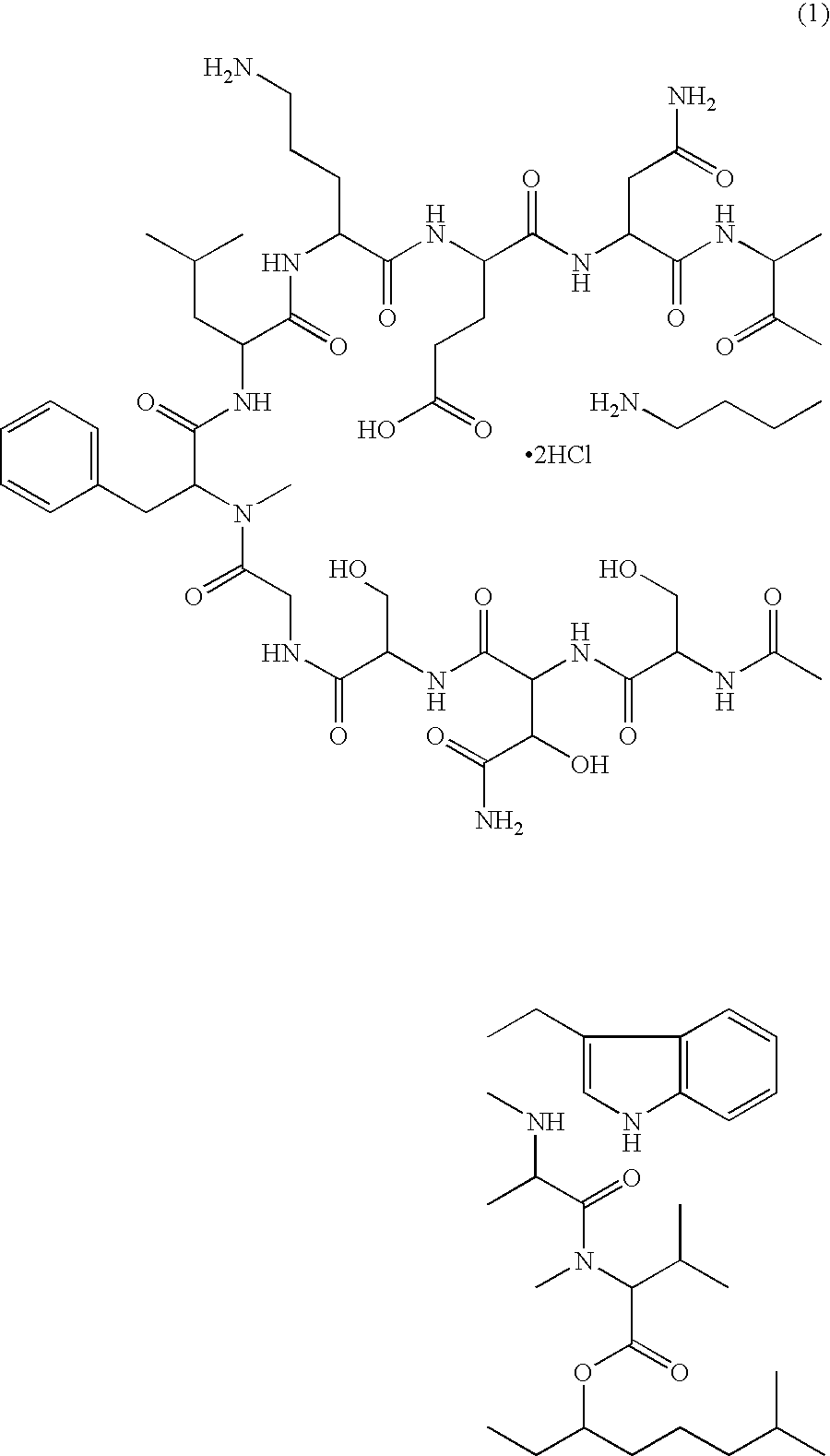
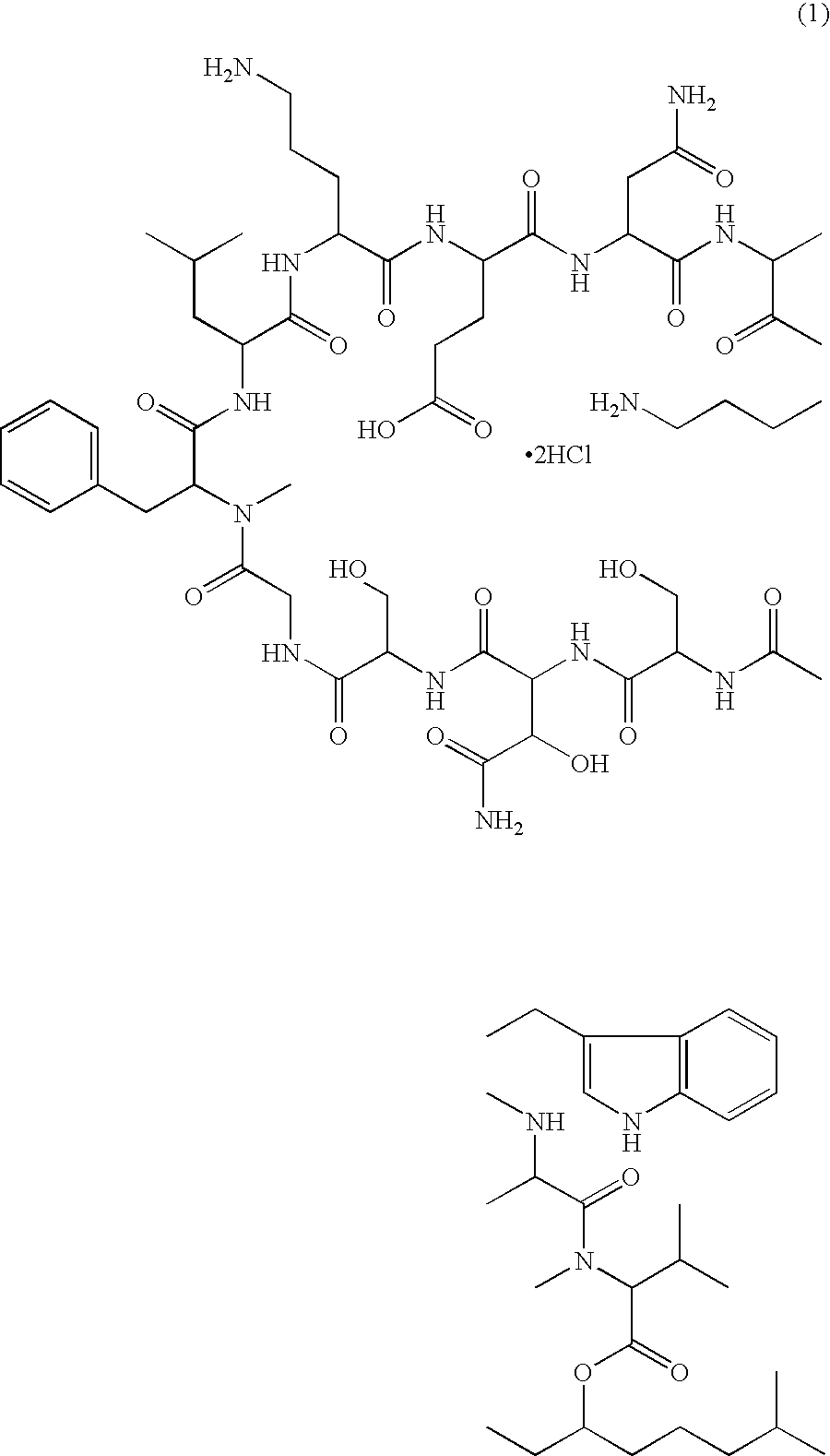
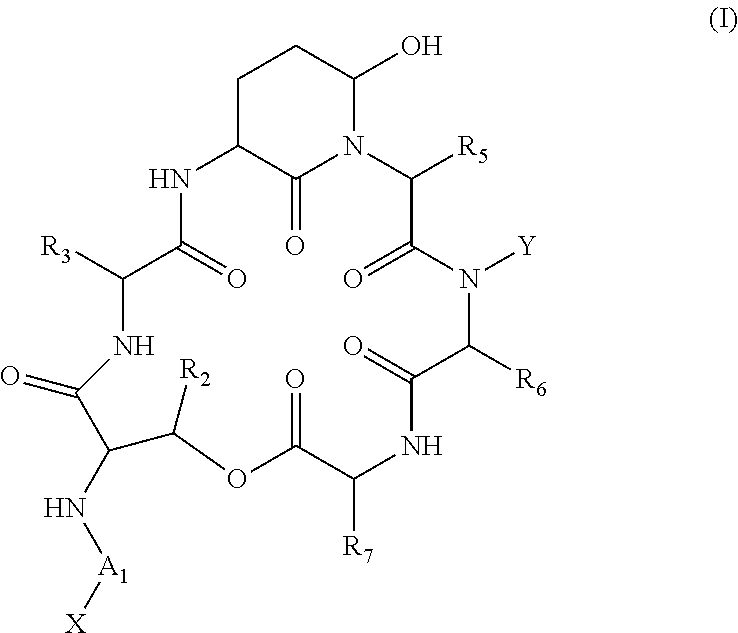
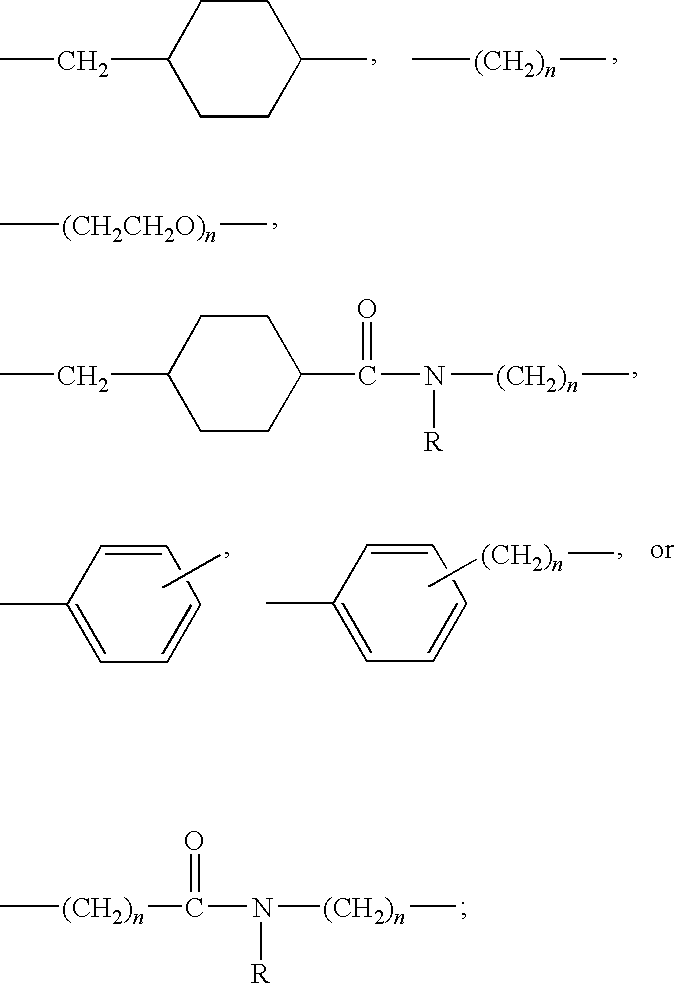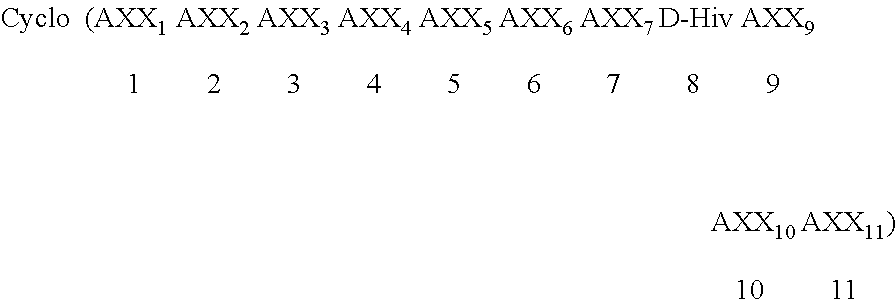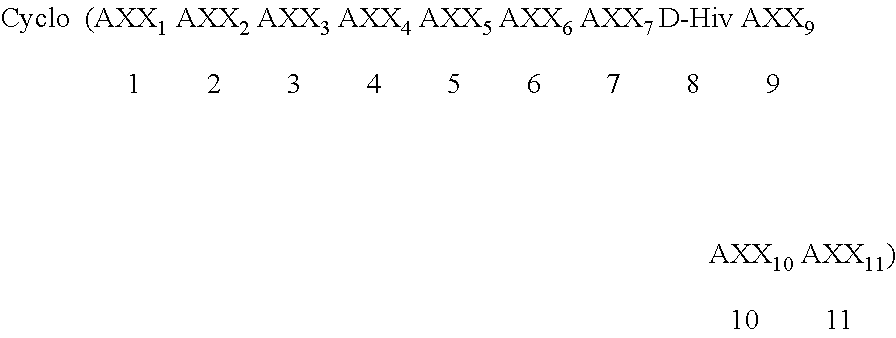Patents
Literature
45 results about "Depsipeptide formation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
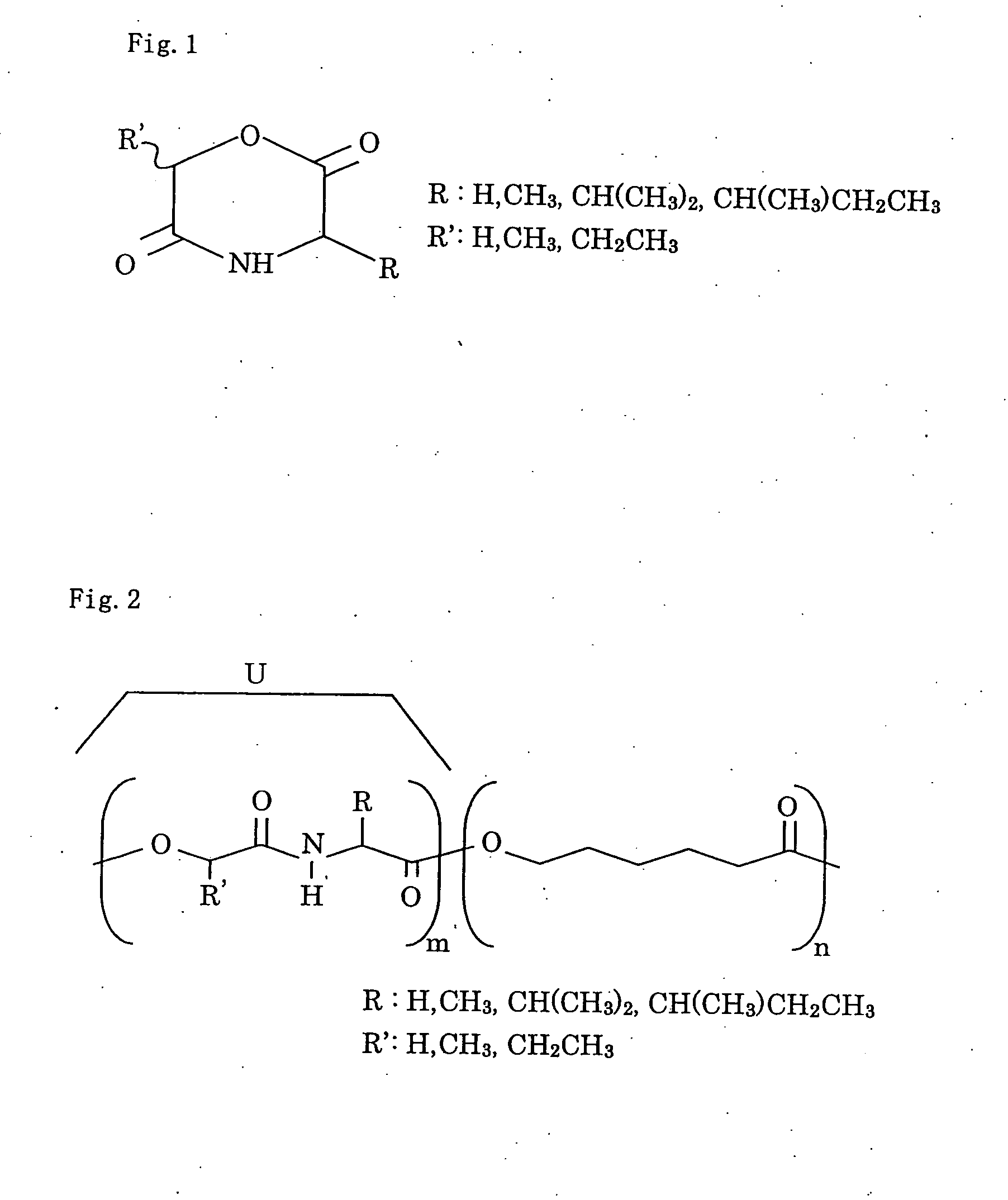
A depsipeptide is a peptide in which one or more of its amide, -C(O)NHR-, groups are replaced by the corresponding ester, -C(O)OR, or more generally, is a molecule that has both peptide and ester linkages in proximity in the same amino acid-containing small molecule or chain.
Macrocyclic depsipeptide antibody-drug conjugates and methods
InactiveUS20090226465A1Prevent proliferationInhibit tumor cell growthImmunoglobulins against animals/humansAntibody ingredientsDiseaseKahalalide F
The present invention relates to antibody-drug conjugate compounds of Formula I: Ab (L D)p I where one or more macrocyclic depsipeptide drug moieties (D), selected from Aplidin, Didemnin B, Kahalalide F, and analogs and derivatives therefrom, are covalently attached by a linker (L) to an antibody (Ab) which binds to one or more tumor-associated antigens or cell-surface receptors. These compounds may be useful in methods of diagnosis or treatment of cancer, and other diseases and disorders.
Owner:GENENTECH INC
Depsipeptide for therapy of kidney cancer
ActiveUS20060135413A1Confirming its antitumor effectBiocideCyclic peptide ingredientsKidney cancerBULK ACTIVE INGREDIENT
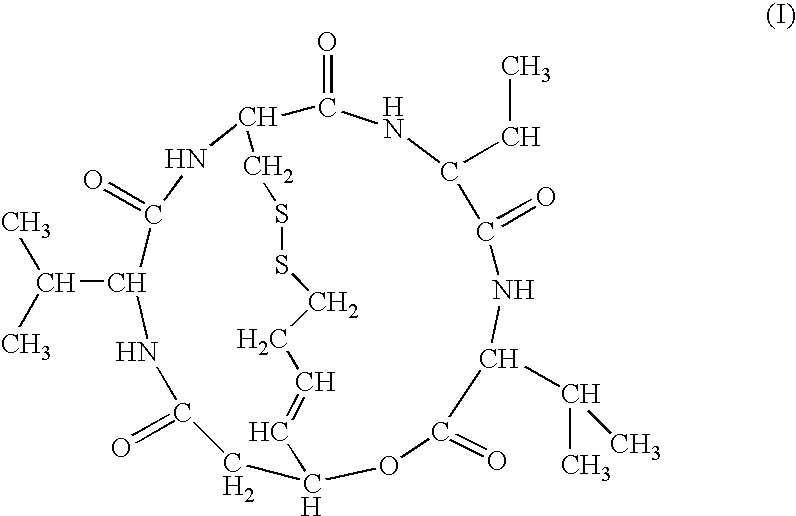
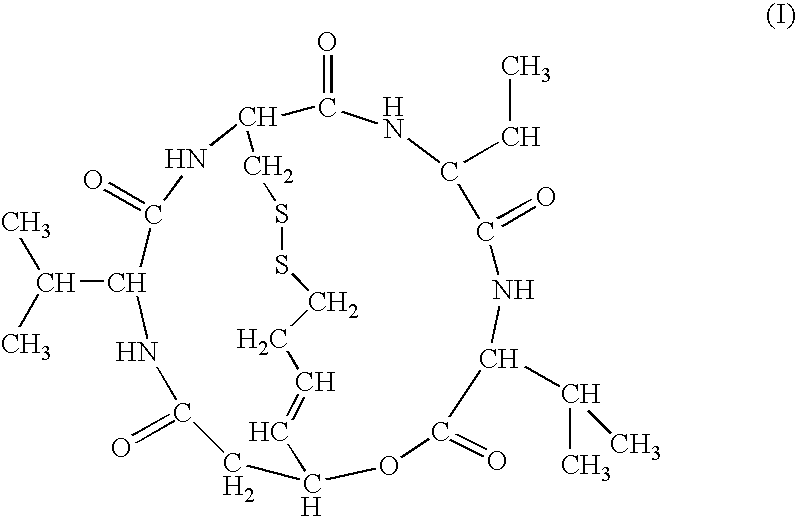
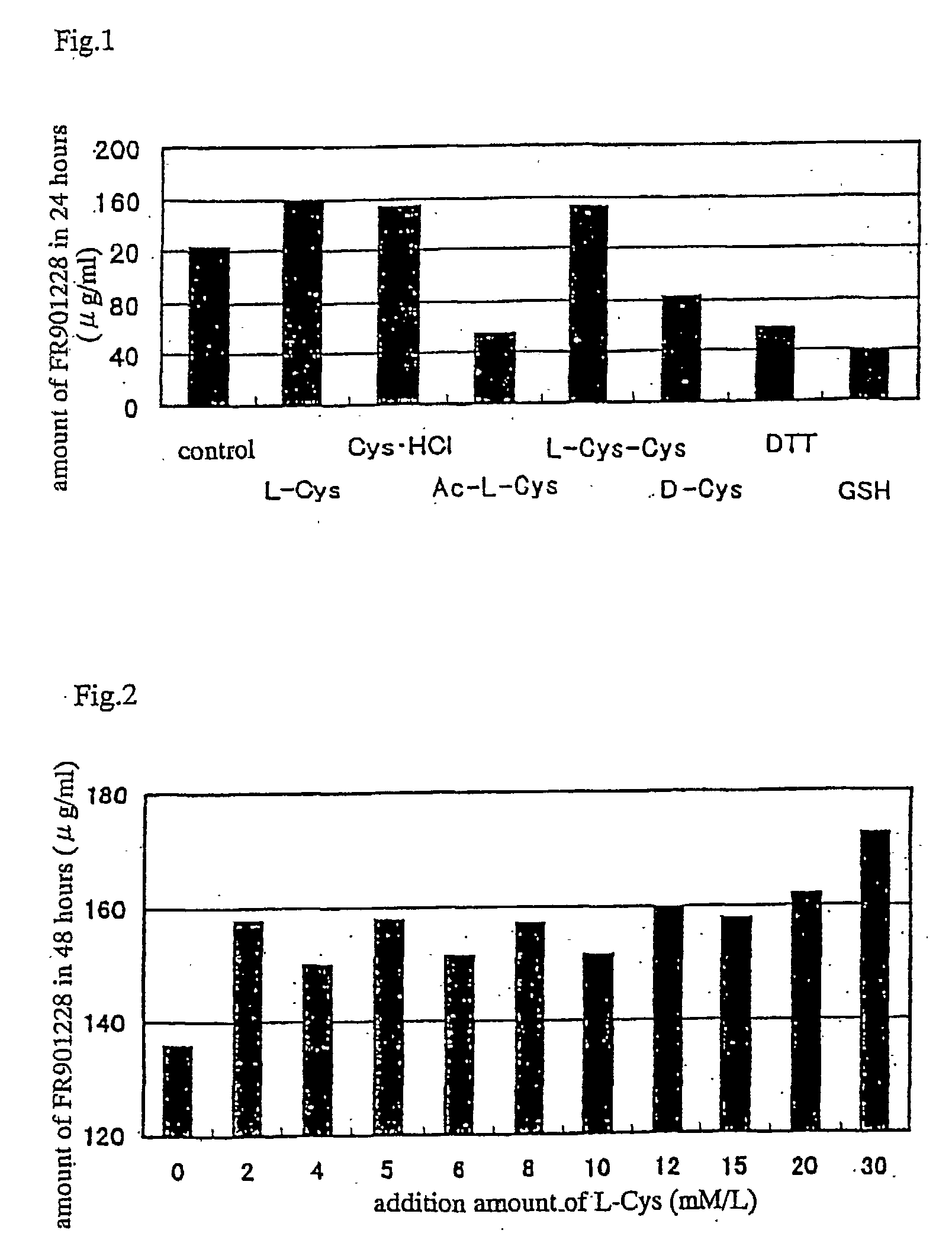
The present invention provides a therapeutic agent of kidney cancer, which comprises FK228 of the formula (I) or a salt thereof. FK228 or a salt thereof, which is an active ingredient in the present invention, shows a superior antitumor activity in vivo against kidney cancer.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE UNITED STATES REPRESENTED BY THE SEC DEPT OF +1
Depsipeptide and congeners thereof for use as immunosuppressants
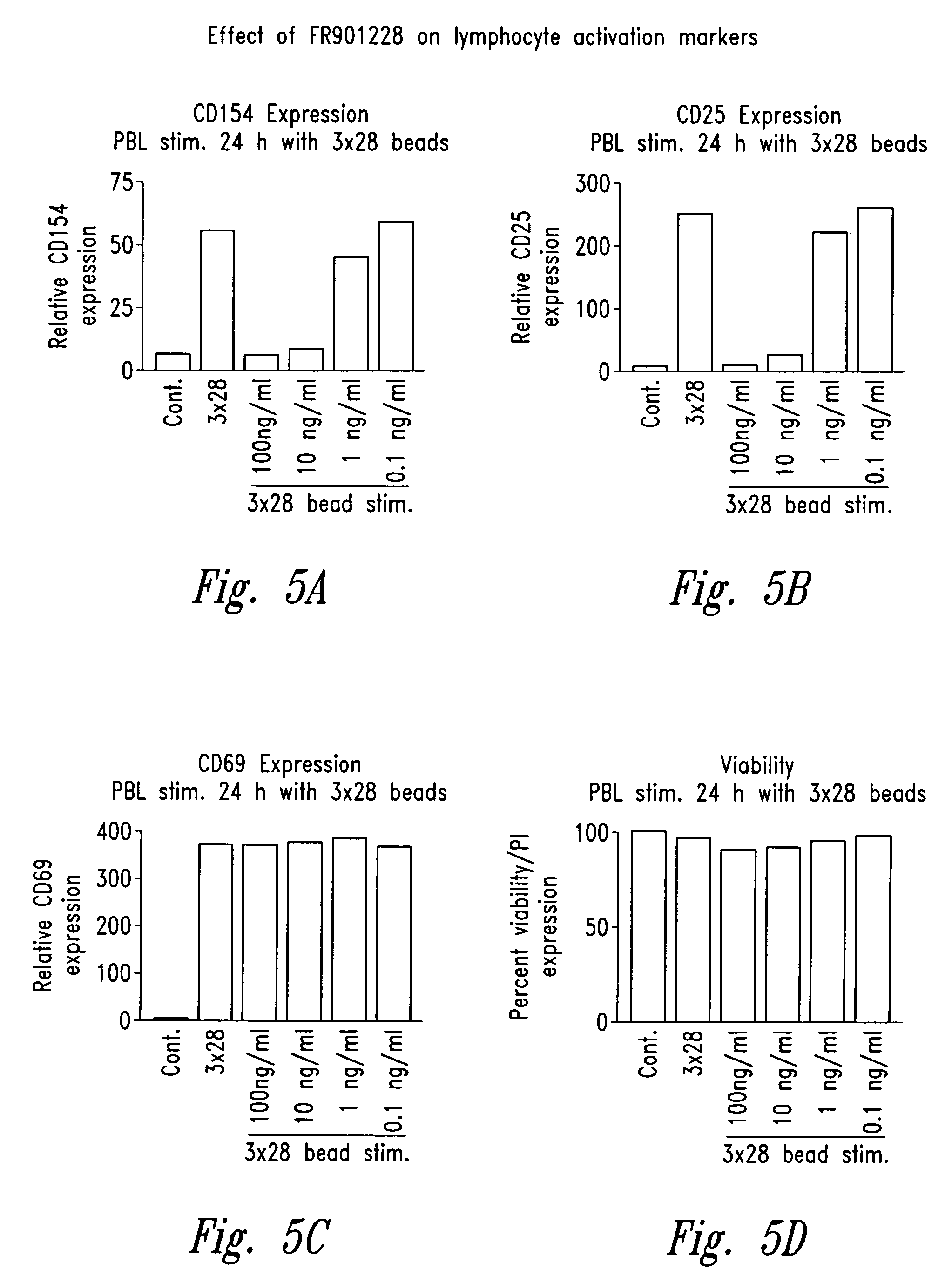
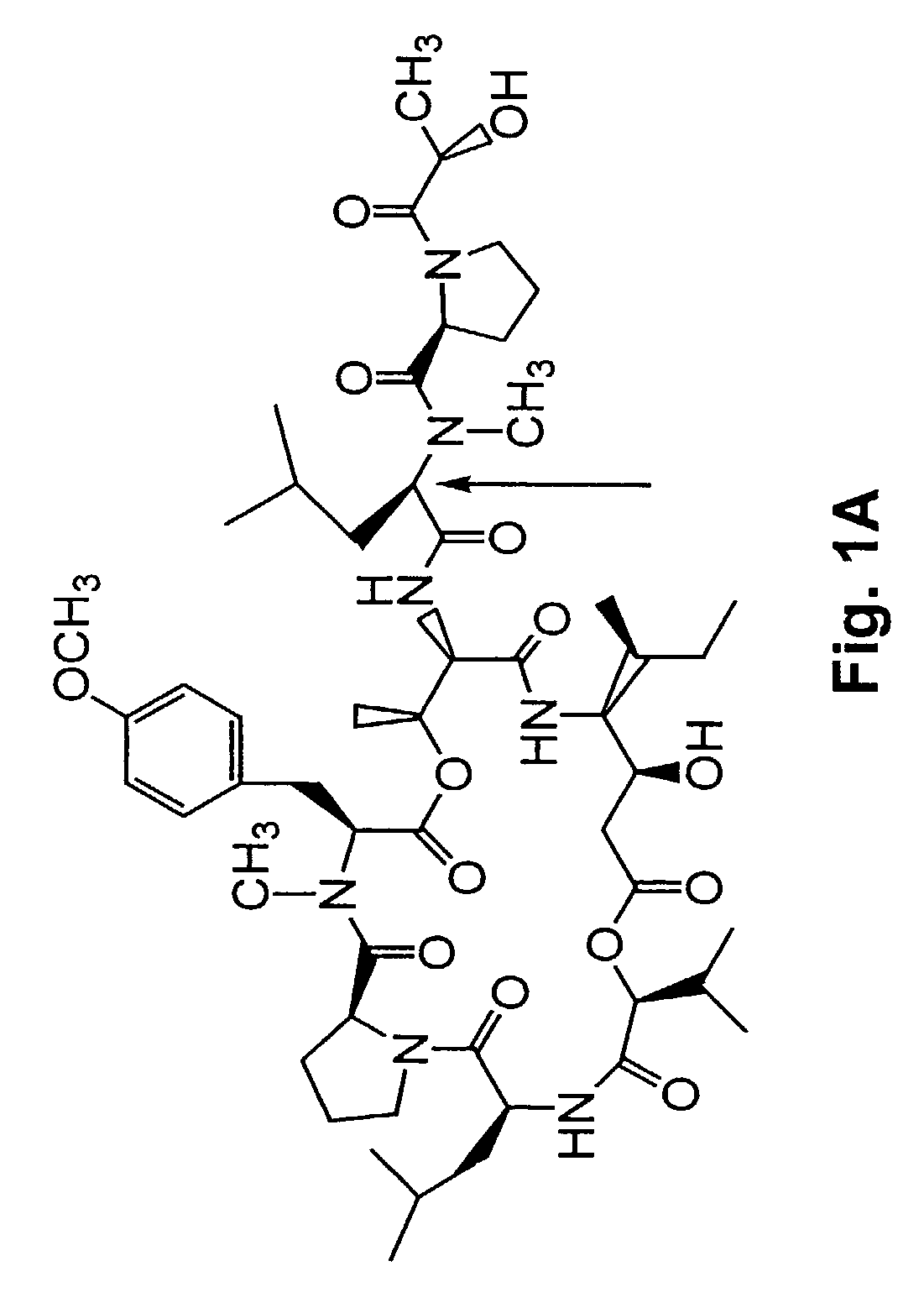
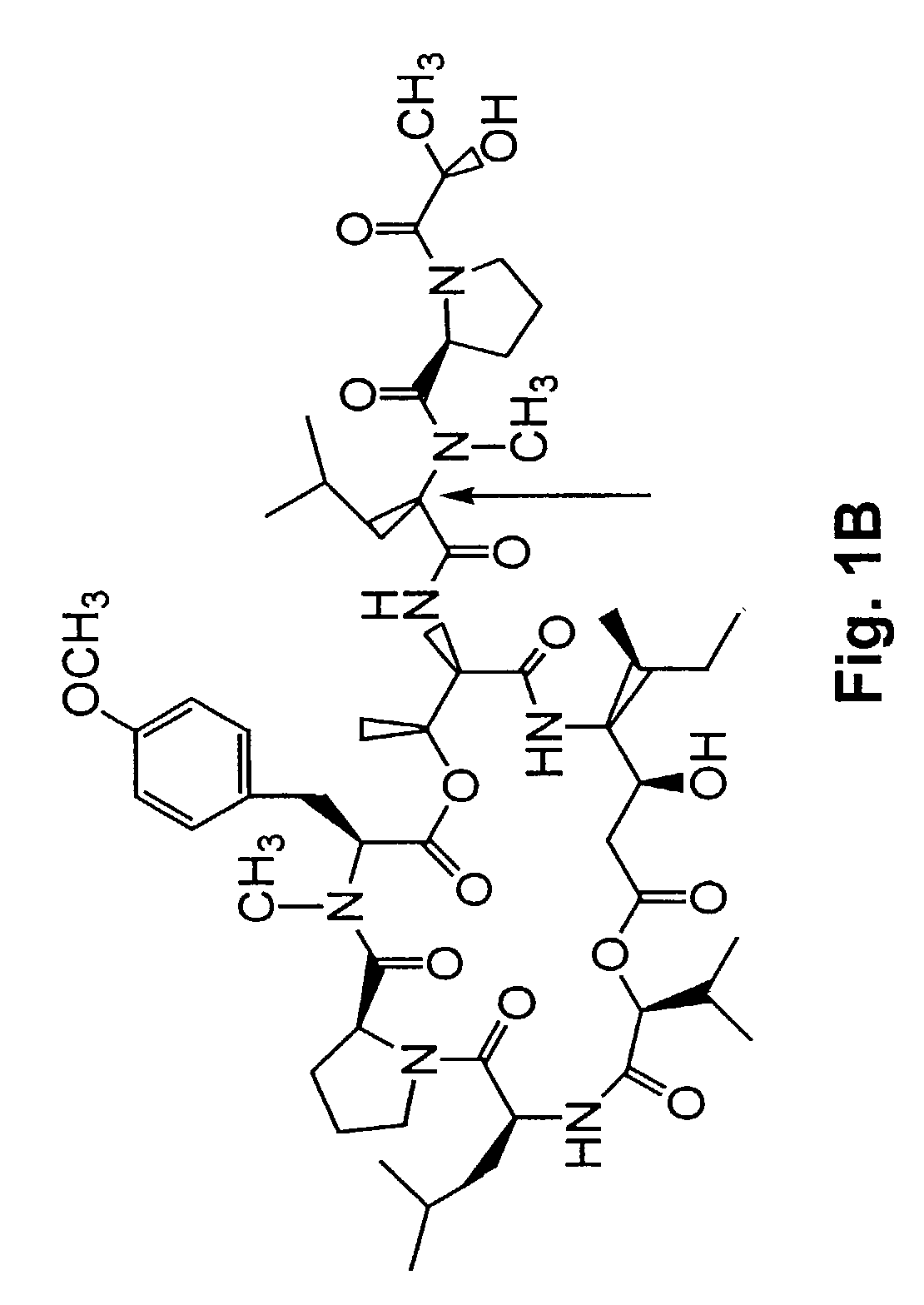
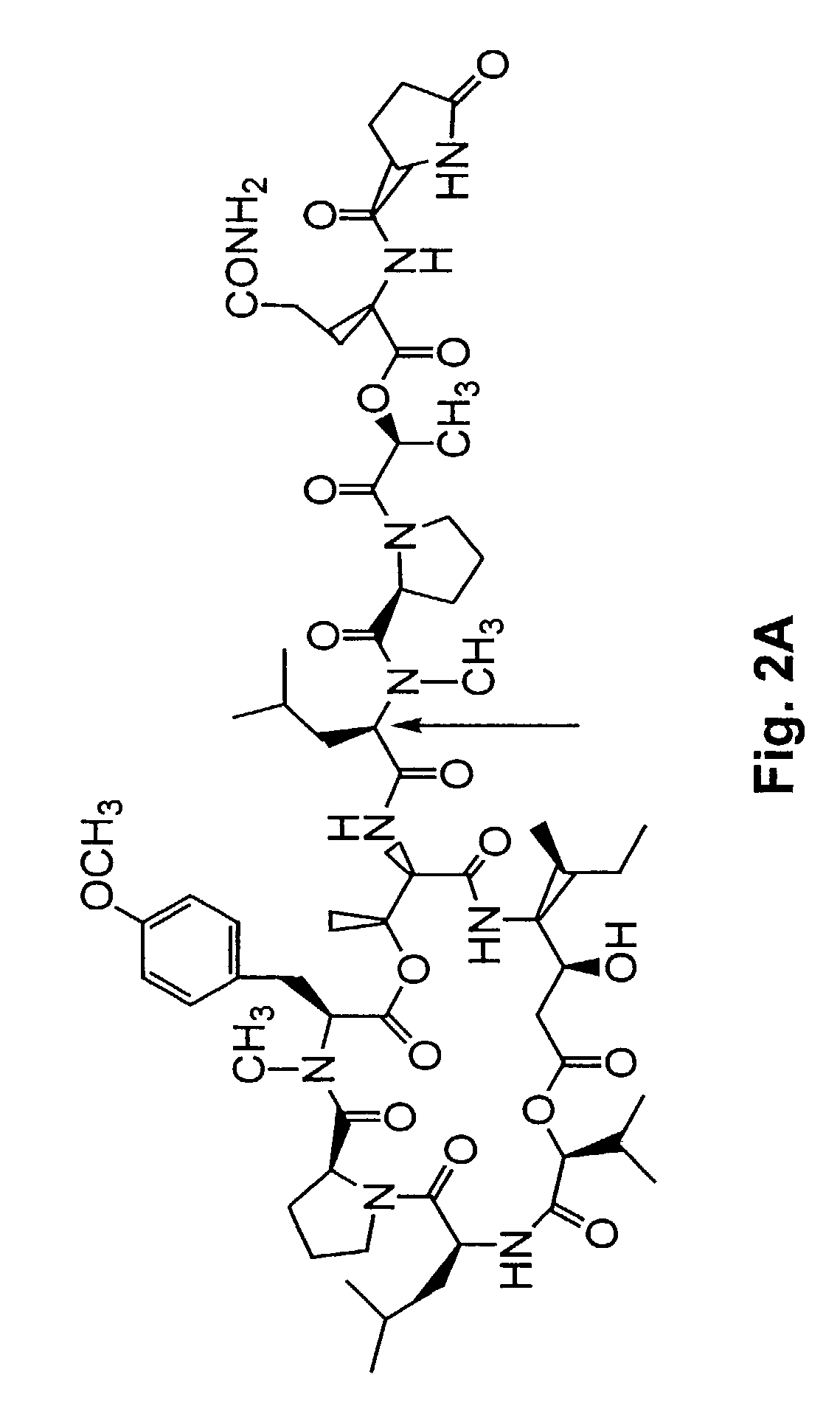
Depsipeptides and congeners thereof are disclosed having the following structure:wherein m, n, p, q, X, R1, R2 and R3 are as defined herein. These compounds, including FR901228, have activity as, for example, immunosuppressants, as well as for the prevention or treatment of patients suffering or at risk of suffering from inflammatory, autoimmune or immune system-related diseases including graft-versus-host disease and enhancement of graft / tissue survival following transplant. Also provided are methods for inhibiting lymphocyte activation, proliferation, and / or suppression of IL-2 secretion.
Owner:CELGENE CORP
Didemnin analogs and fragments and methods of making and using them
The present invention relates to macrocyclic depsipeptides, including didemnin analogs and fragments thereof, which are useful as anti-cancer agents and for other purposes. The invention includes numerous didemnin analogs and fragments and methods of making them. Methods of using these compounds as inhibitors of protein synthesis, cell growth, and tumorigenesis and as enhancers of apoptosis are also provided.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Biodegradable bio-absorbable material for clinical practice and method for producing the same
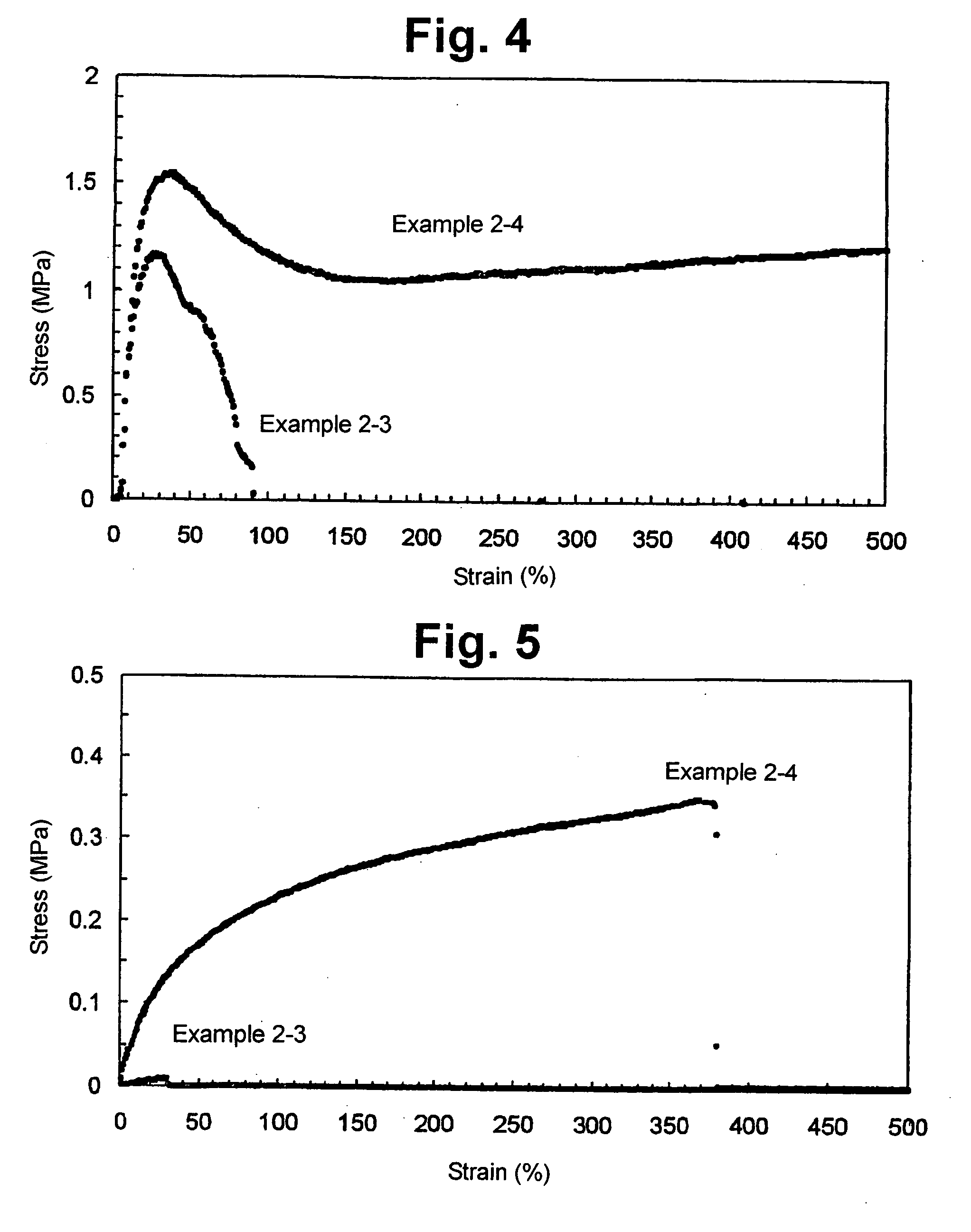
InactiveUS20050163822A1Deteriorate dynamic propertySuture equipmentsCyclic peptide ingredientsAbsorbable polymersUltimate tensile strength
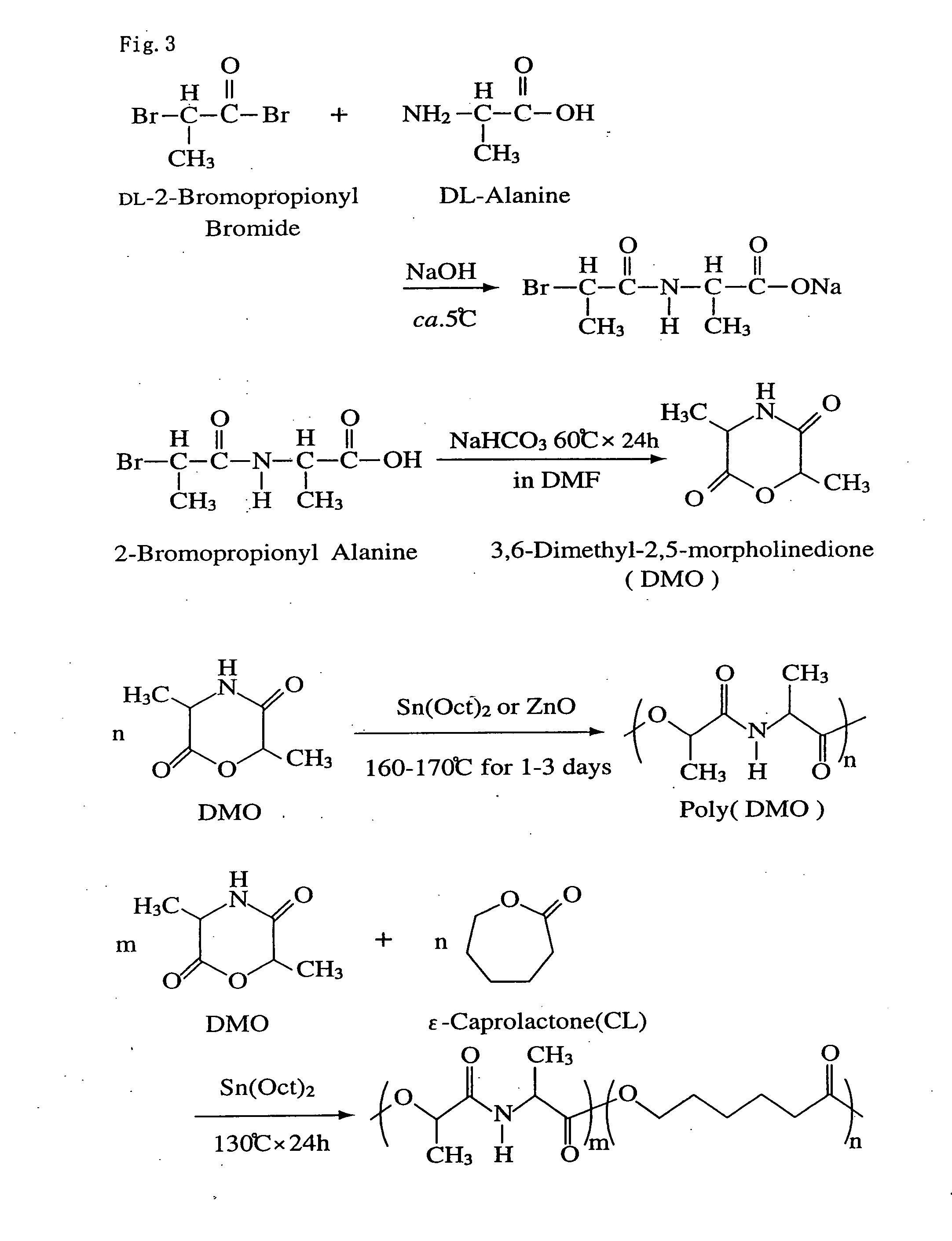
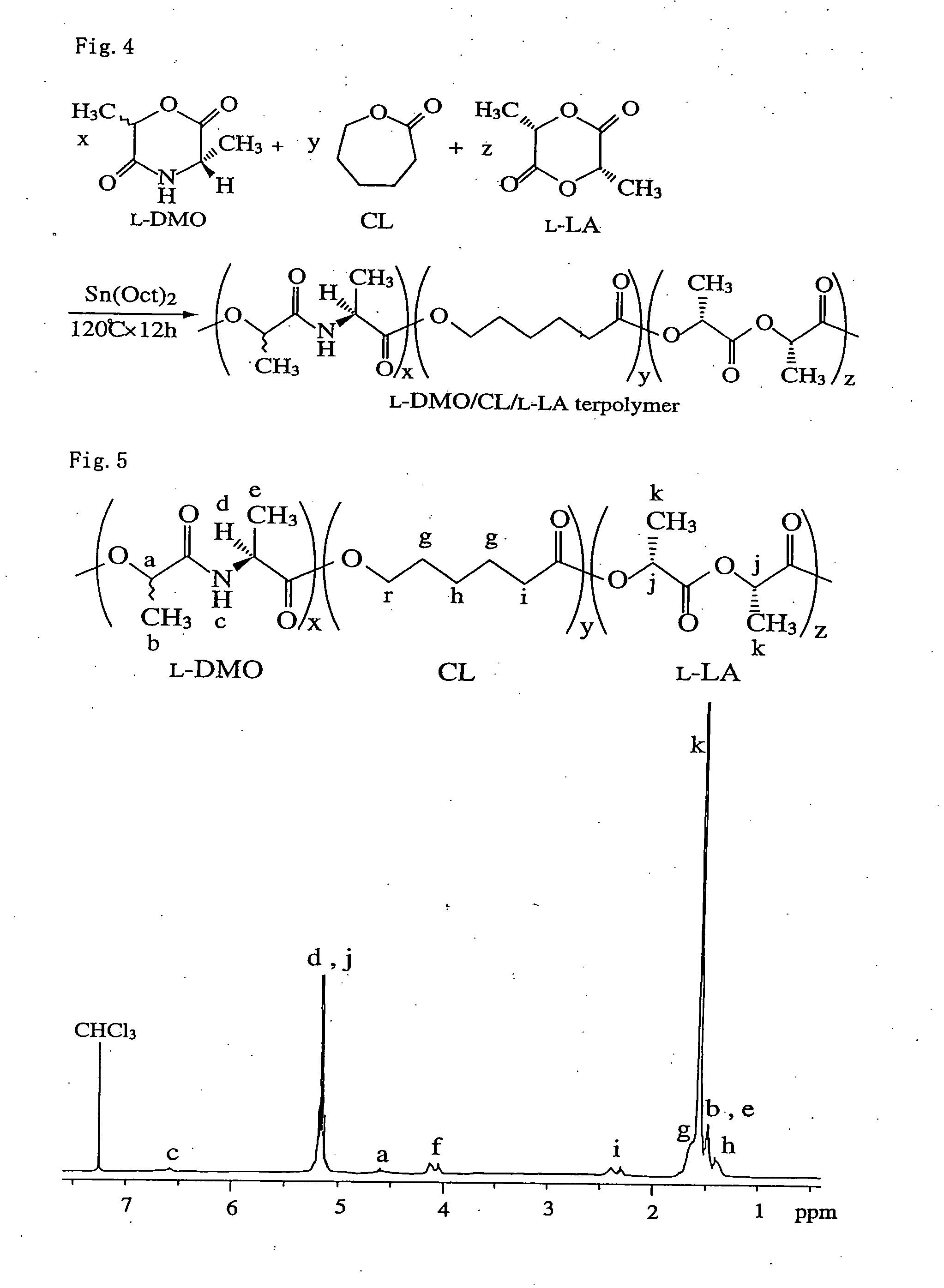
Bio-absorbable polymers such as vascular stent and suture thread for use as materials for clinical practice have almost definite dynamic properties such as tensile strength and degradation rate for absorption. When the dynamic properties thereof are elevated, therefore, the bio-absorbable polymers turn fragile, involving slower degradation rate. When the degradation rate is elevated, further, the dynamic properties are deteriorated. Disadvantageously, such bio-absorbable polymers have limited purposes for use and limited sites for use. Thus, copolymerization of bio-absorbable polymers with a cyclic depsipeptide to form a copolymer of the ring-opened and copolymerized depsipeptide can allow the adjustment of the dynamic properties and degradation rate of the resulting copolymer depending on the content of the depsipeptide.
Owner:GOODMAN & COMPANY
Antiinfective Lipopeptides
The present invention relates to novel depsipeptide compounds. The invention also relates to pharmaceutical compositions of these compounds and methods of using these compounds as antibacterial compounds. The invention also relates to methods of producing these novel depsipeptide compounds and intermediates used in producing these compounds.
Owner:CUBIST PHARMA INC
Method of producing fr901228
InactiveUS20030186388A1Increase productionEfficient productionAntibacterial agentsMicroorganism based processesSecretionSecreted substance
Depsipeptides and congeners thereof are disclosed having structure (I), wherein m, n, p, q, X, R1, R2 and R3 are as defined herein. These compounds, including FR901228, have activity as, for example, immunosuppressants, as well as for the prevention or treatment of patients suffering or at risk of suffering from inflammatory, autoimmune or immune system-related diseases including graft-versus-host disease and enhancement of graft / tissue survival following transplant. Also provided are methods for inhibiting lymphocyte activation, proliferation, and / or suppression of IL-2 secretion.
Owner:ASTELLAS PHARMA INC
Depsipeptide and uses thereof
Owner:NOVOBIOTIC PHARMA LLC
Solution Phase Processes for the Manufacture of Macrocyclic Depsipeptides and New Intermediates
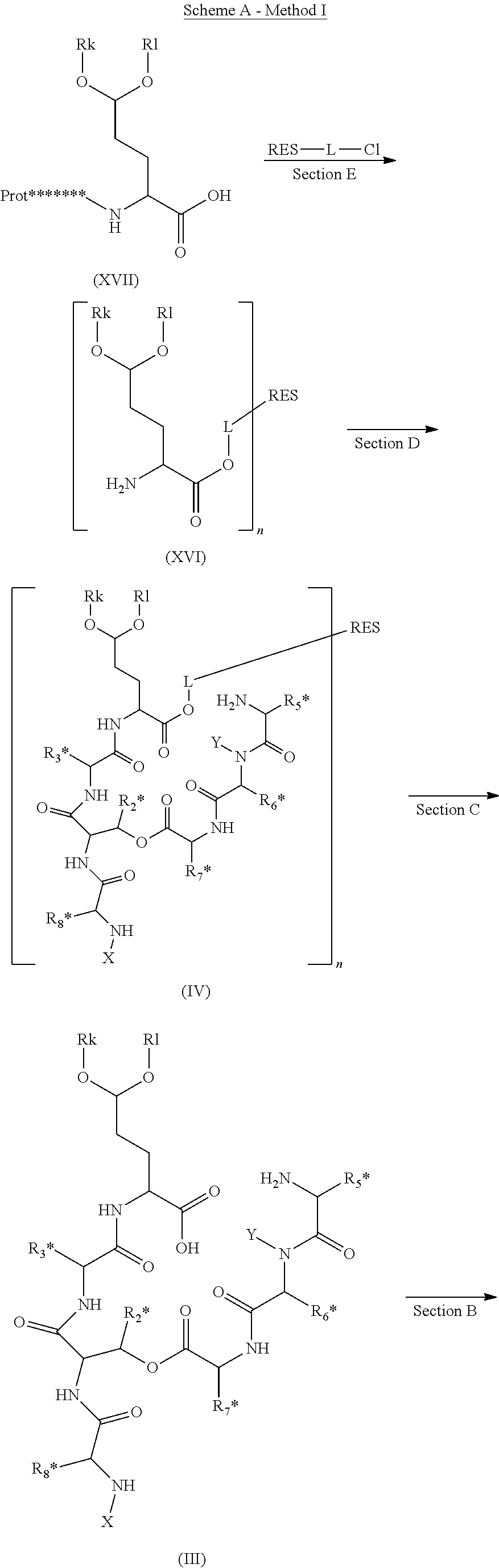
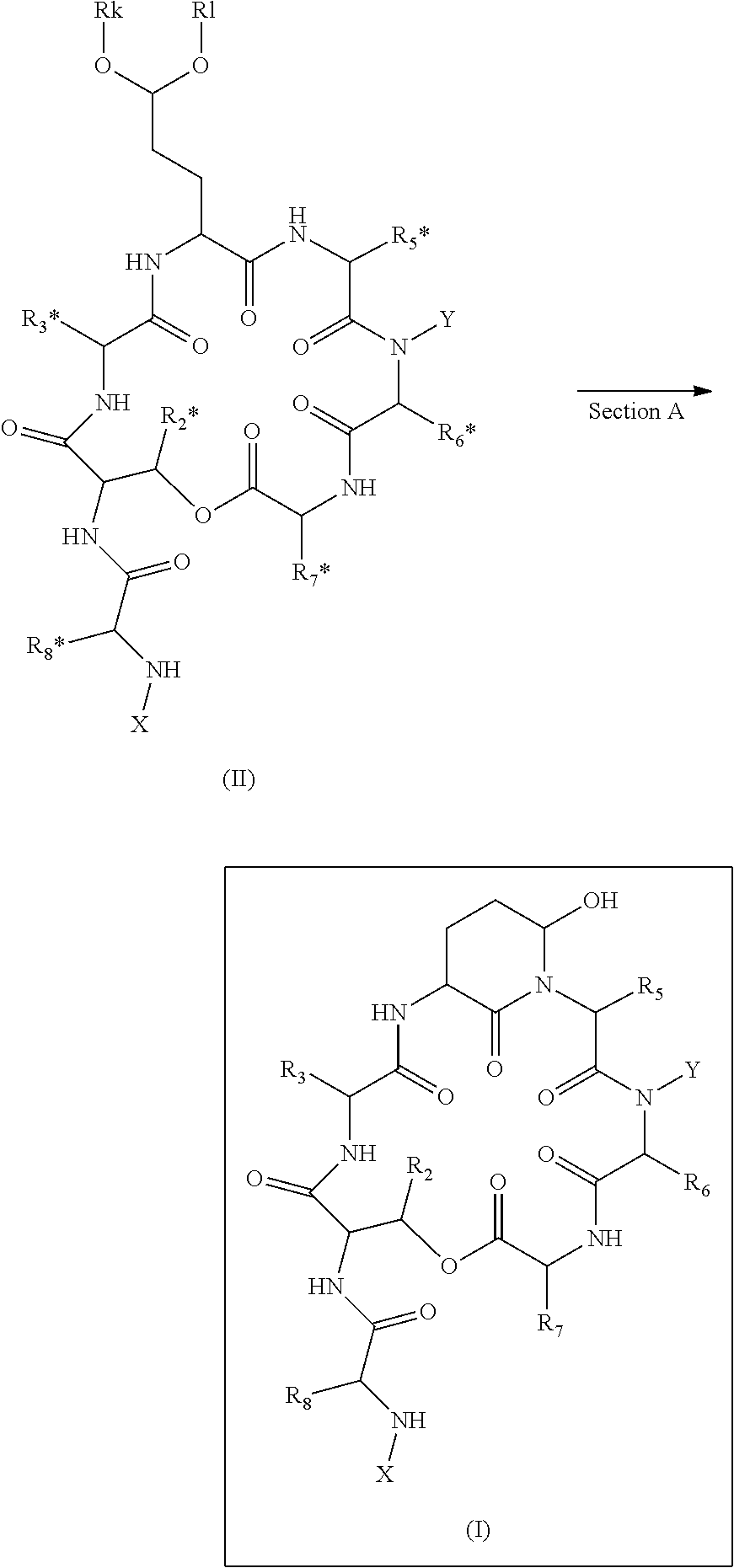
ActiveUS20140100355A1High purityHigh yieldPeptide/protein ingredientsDepsipeptidesCompound (substance)Depsipeptide
The invention relates to a method or process for solution phase chemical manufacture of depsipeptides of the formula I,wherein the symbols have the meaning defined in the description, to new intermediates and their manufacture, as well as related invention embodiments.
Owner:NOVARTIS AG
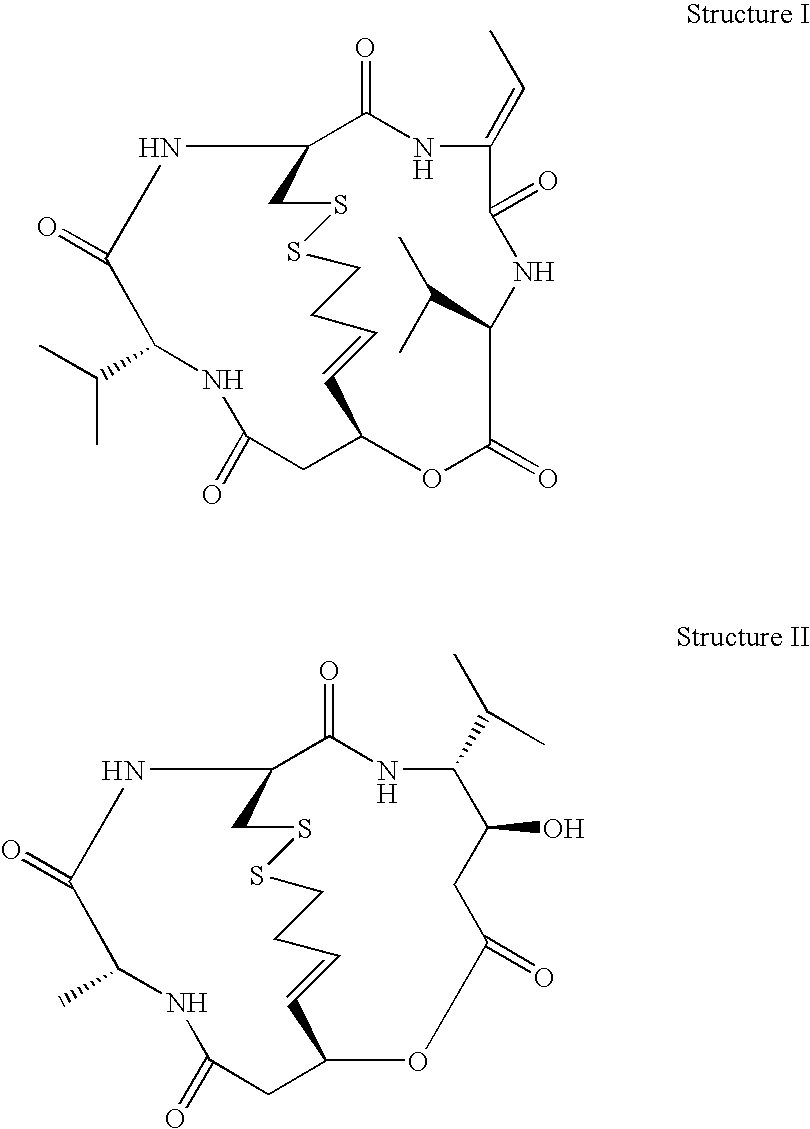
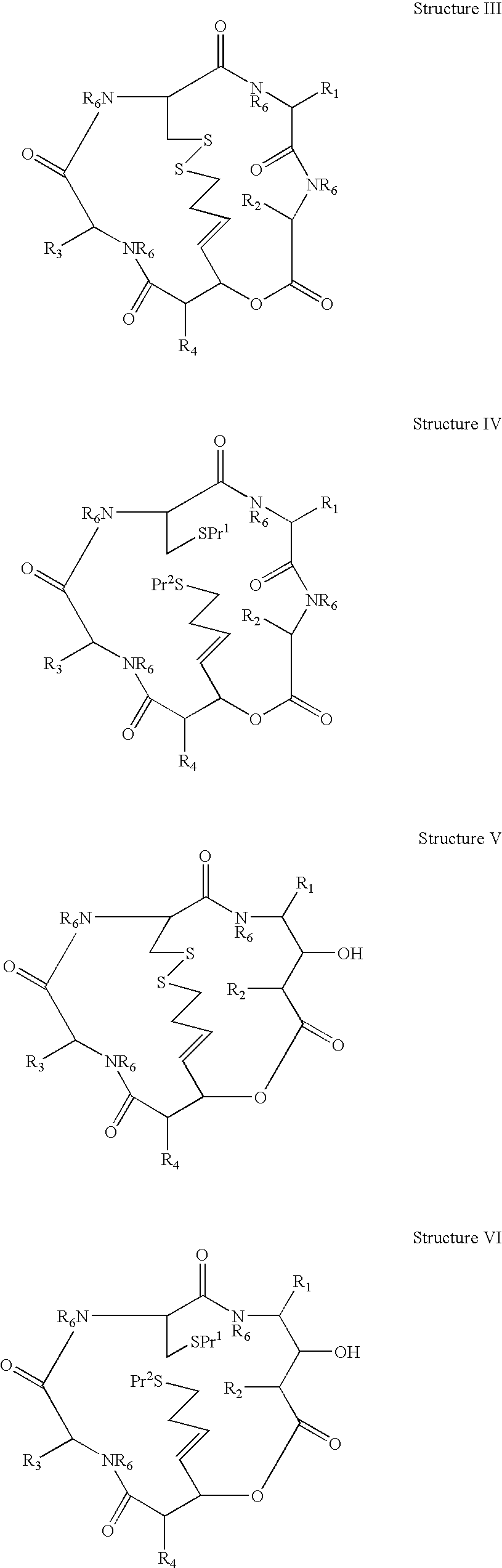
Depsipeptides and Their Therapeutic Use
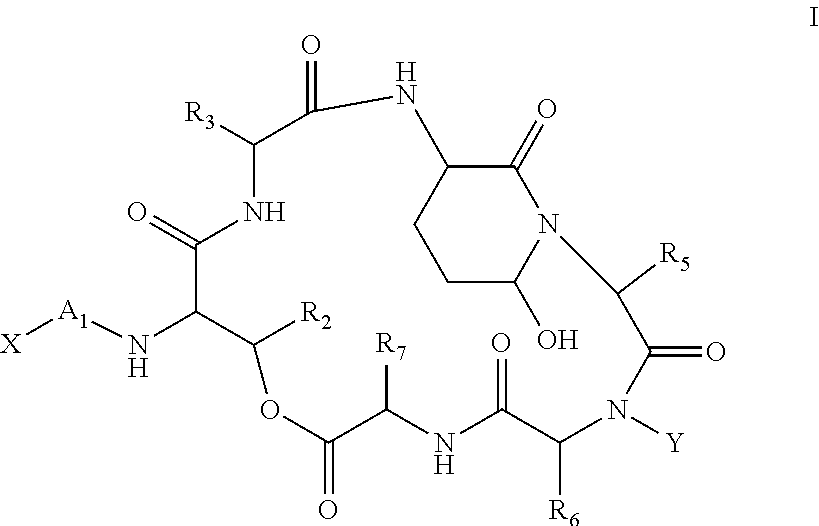
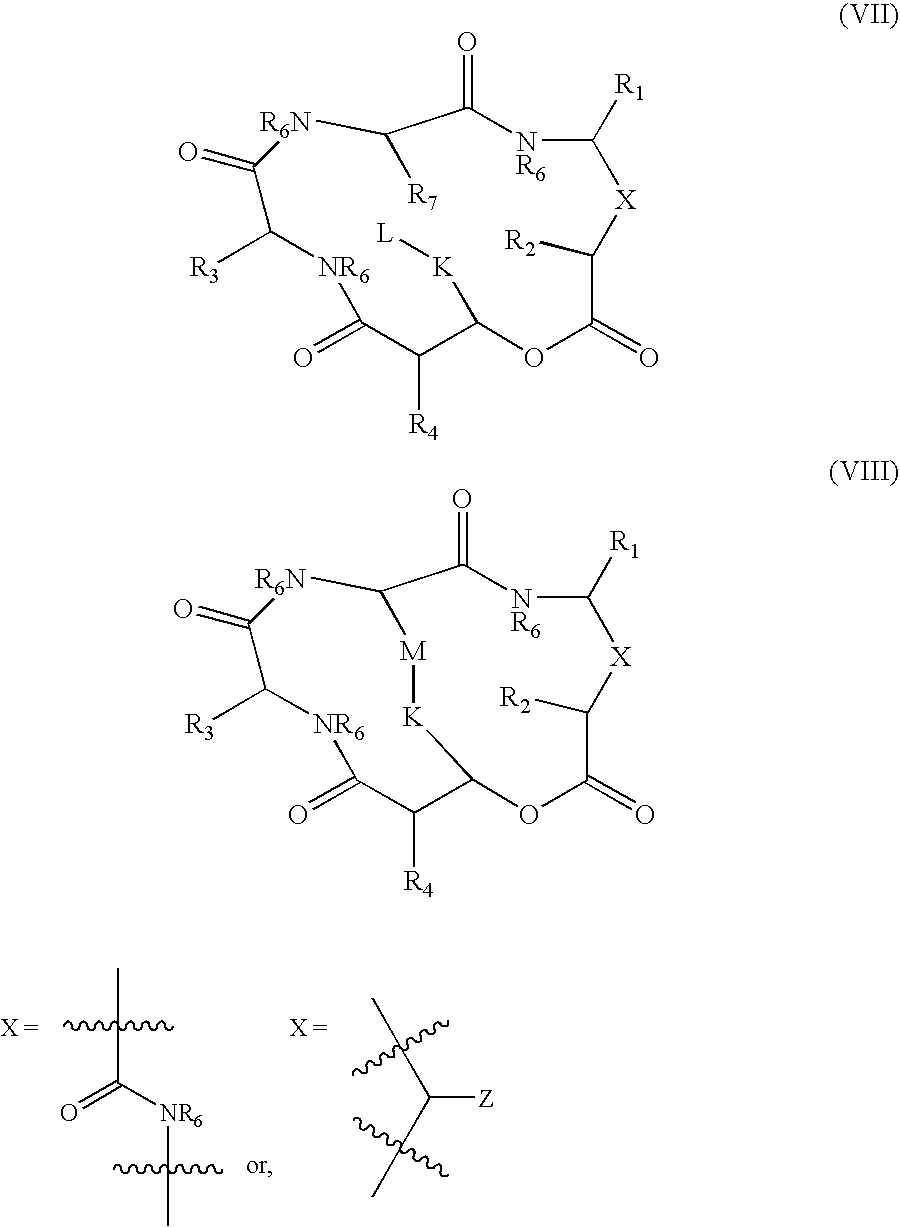
A compound of the general Structure (VII) or (VIII) including isoteres and pharmaceutically acceptable salts thereof, wherein R1, R2 (where X=—CONR6—), R3 and R7 are the same or different and each represents an amino-acid side chain moiety; R2 (where X=—CHZ—), R4 and R6 are the same or different and each represents hydrogen, C1-C6 alkyl, C2-C6 alkenyl, or C2-C6 alkynyl; K represents a linear or branched chain of carbon atoms and containing 1-10 atoms; L represents a moiety capable of chelating zinc in the active site of a histone deacetylase (HDAC) or of conversion to such a moiety in vivo (by hydrolysis or reduction, for example); M is a linear or branched chain of carbon or other atoms and containing 1-10 atoms, and capable of undergoing in vivo cleavage to give Structure (VII); and Z is a heteroatom bonded to the macrocycle by a single or double bond, and any other group bonded to Z is H or a protecting group.
Owner:KARUS THERAPEUTIC LTD
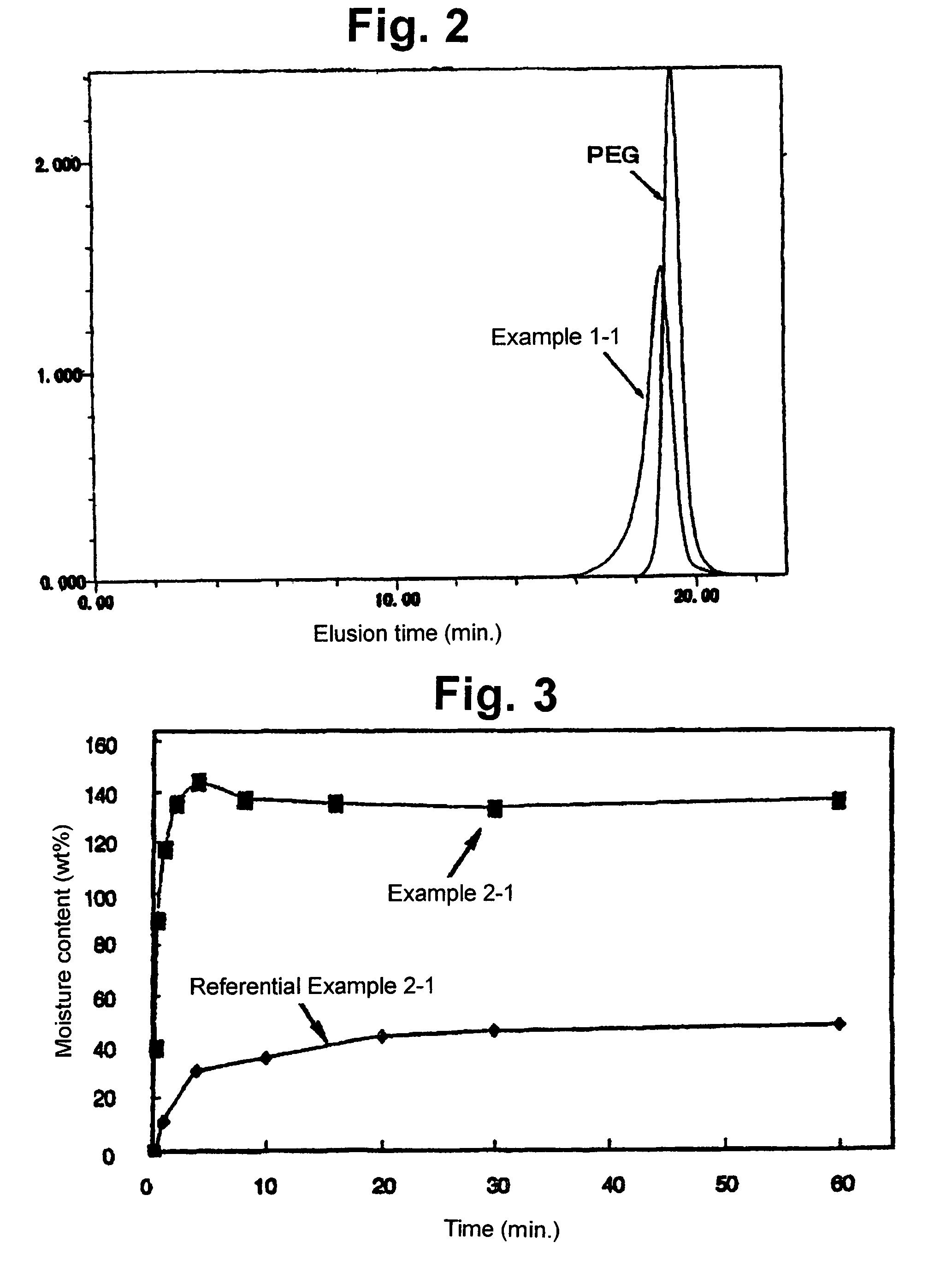
Triblock copolymer, method for producing the same, and biocompatible material
InactiveUS7714077B2Promote absorptionIncrease flexibilityPowder deliveryProsthesisPolymer scienceLactide
The present invention relates to a triblock copolymer, that is multipurpose yet has sufficient properties particularly for medical applications, and is useful as a material having excellent flexibility and water absorbability, as well as to a method for producing the same, and a biocompatible material. The copolymer of the present invention is composed of segments A1 and A2 each composed of a polymer having a depsipeptide unit, such as a segment selected from a homopolymer of depsipeptide or a copolymer of lactide and depsipeptide, and segment B composed of polyalkylene glycol, such as PEG, and is a A1-B-A2 triblock copolymer having a number average molecular weight of 8000 to 500000. The biocompatible material of the present invention contains the triblock copolymer as a main component, and may be used as a tissue anti-adhesion barrier.
Owner:NOF CORP
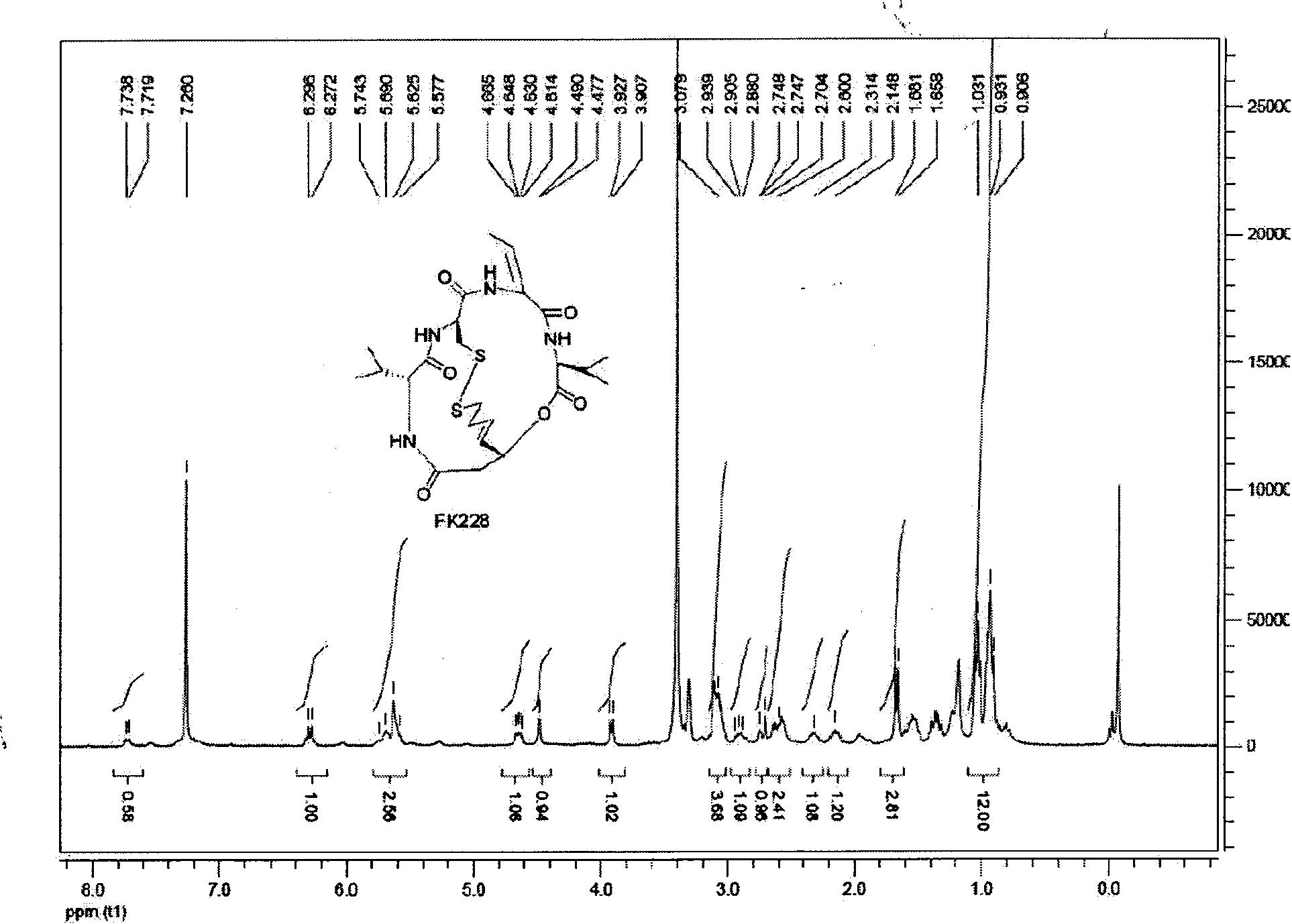
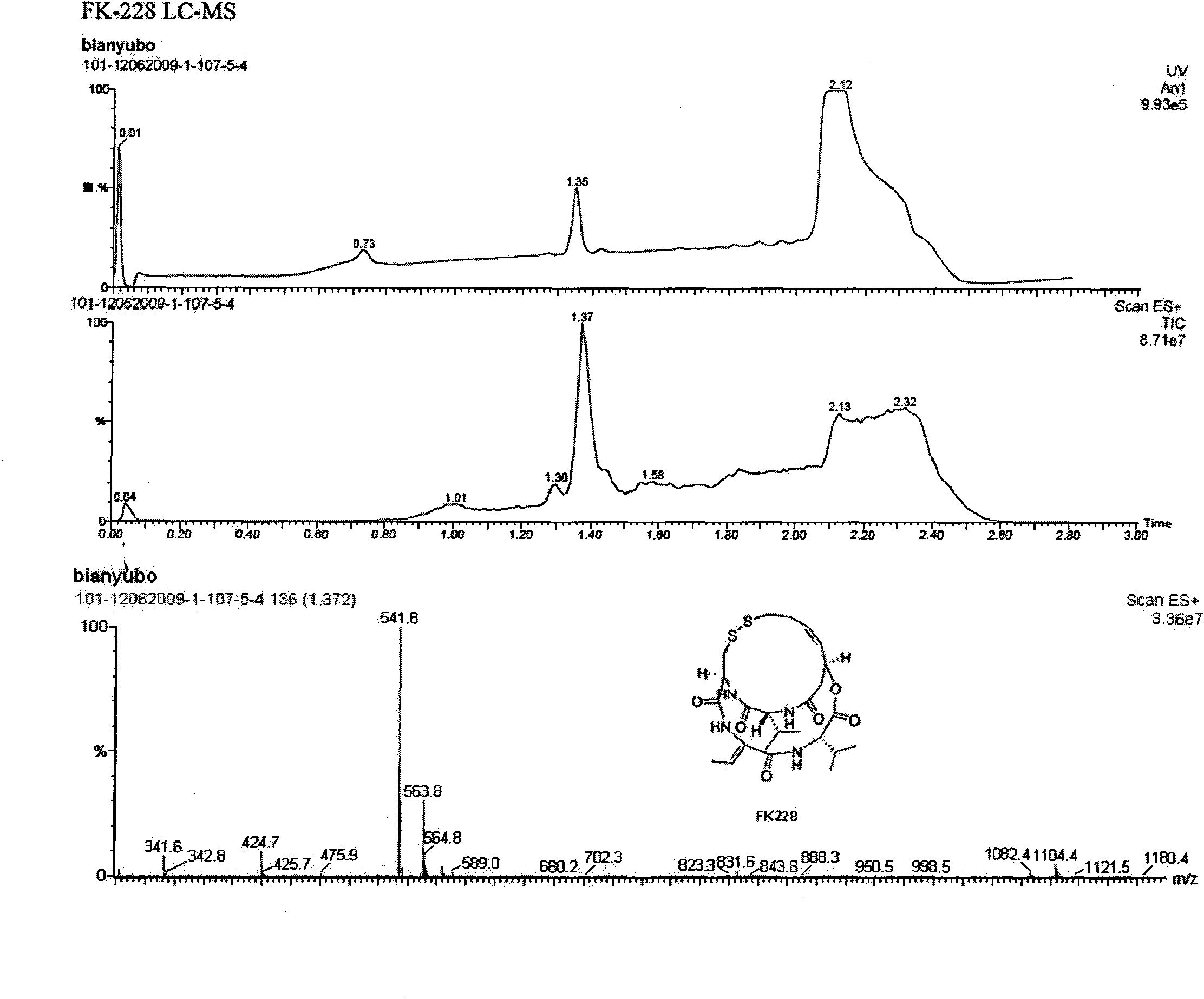
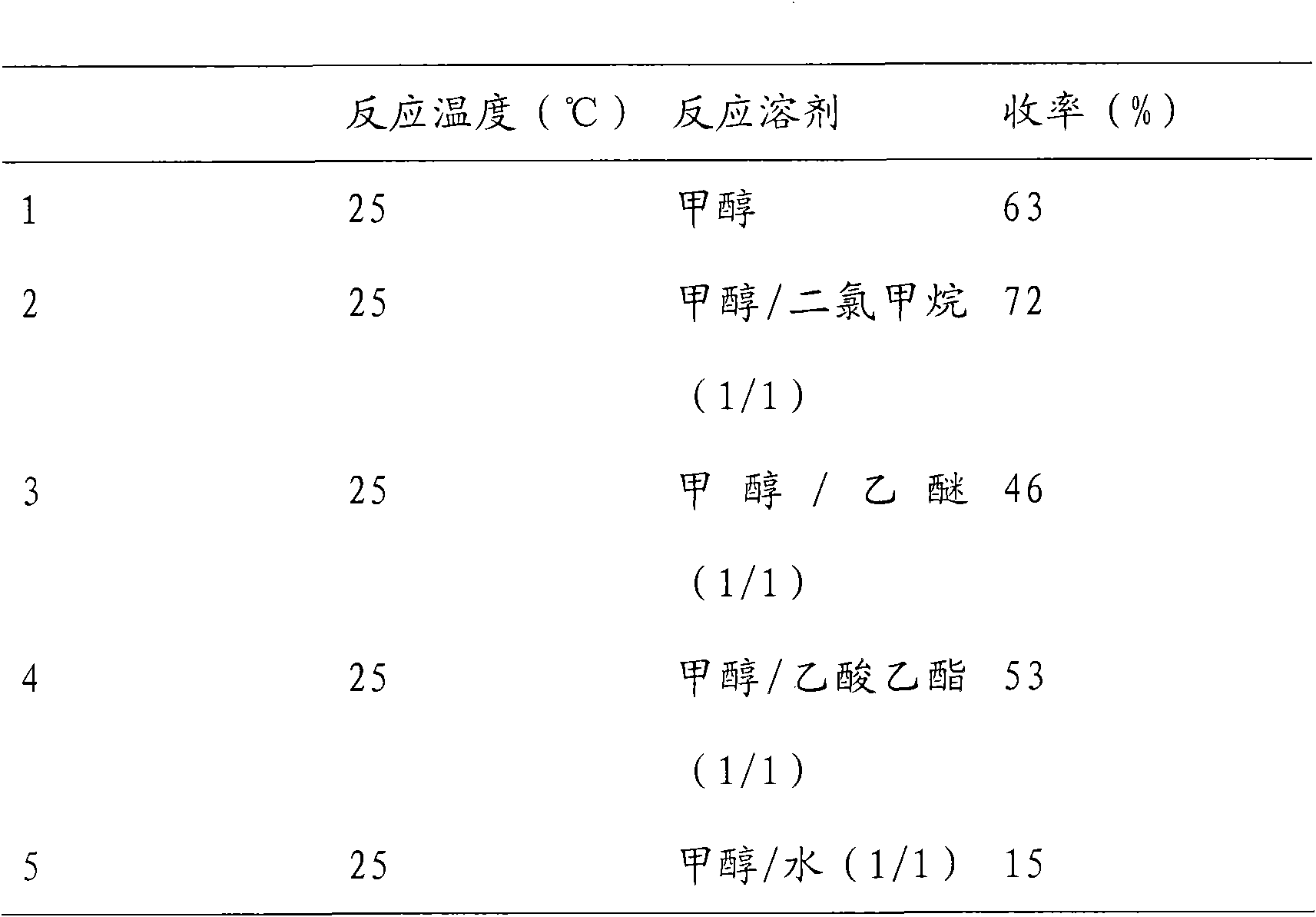
Preparation method of histone deacetylase inhibitor FK228
The invention discloses a new method for preparing depsipeptide FK228, comprising the following steps of: firstly forming annular polypeptide in a ring-closing way through the condensation reaction of an amido bond; and then forming the coupling of sulfide-sulfide bonds through radical reaction catalyzed by iodine by using a mixed solution of methylene dichloride and methanol as a solvent so as to synthesize the FK228. The preparation method of the FK228 has the advantages of easy operation and low cost, primarily discloses a process for preparing gram-grade FK228, greatly enhances the yield compared with past literatures and patents and provides new selection for the preparation and the production of the FK228.
Owner:无锡好芳德药业有限公司
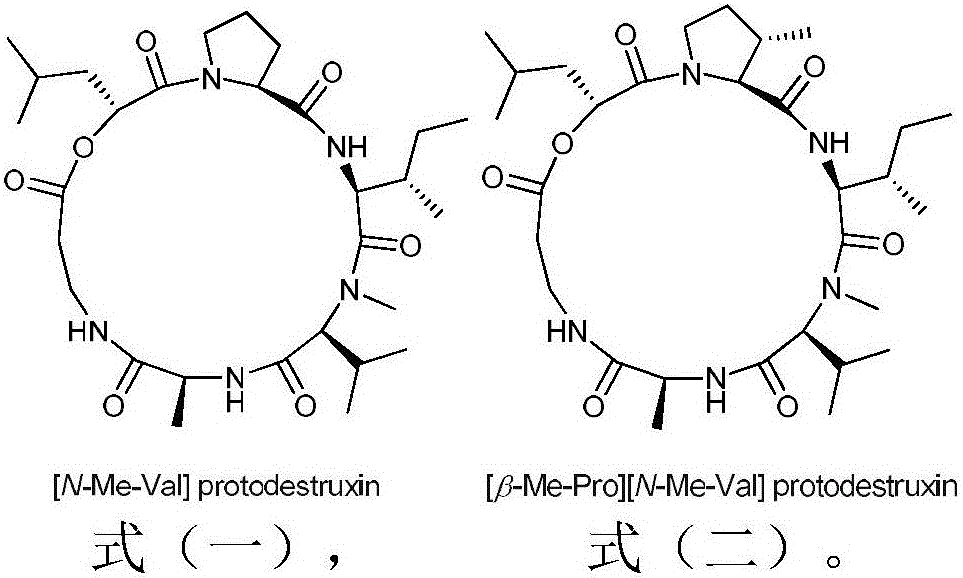
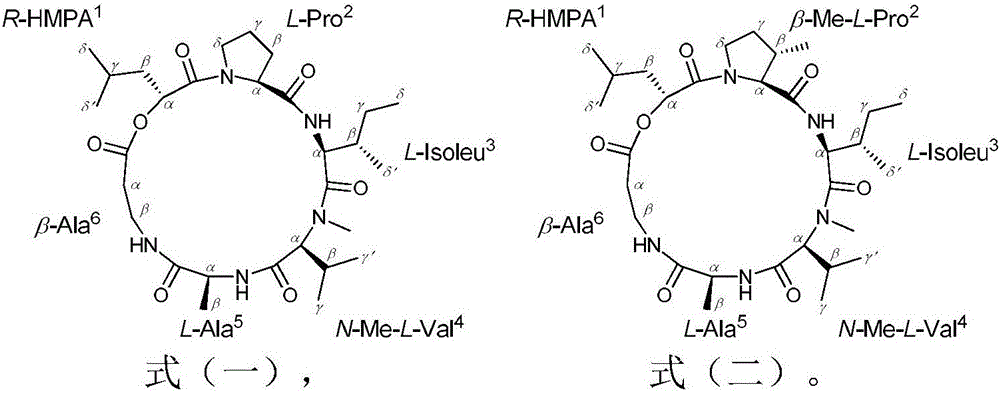
Destruxin depsipeptide derivative and preparation method and application thereof
ActiveCN106810601AHigh insecticidal activitySimple production processBiocideMicroorganism based processesMicroorganismChemical structure
The invention relates to microbial-source pesticide, in particular to a Destruxin depsipeptide derivative and a preparation method and application thereof. The Destruxin depsipeptide derivative is as shown in formula (I) and formula (II). The Destruxin depsipeptide derivative is prepared by the strain BeauveriafelinaAS-70 through fermentation, culture, extraction and separation. By authentication using technologies such as nuclear magnetic resonance and mass spectrum, the chemical structure of the Destruxin depsipeptide derivative is good in pesticide activity.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Tertiary block copolymer, process for producing the same, and biocompatible material
InactiveUS20060223975A1Promote absorptionIncrease flexibilityPowder deliveryProsthesisPolymer scienceLactide
The present invention relates to a triblock copolymer, that is multipurpose yet has sufficient properties particularly for medical applications, and is useful as a material having excellent flexibility and water absorbability, as well as to a method for producing the same, and a biocompatible material. The copolymer of the present invention is composed of segments A1 and A2 each composed of a polymer having a depsipeptide unit, such as a segment selected from a homopolymer of depsipeptide or a copolymer of lactide and depsipeptide, and segment B composed of polyalkylene glycol, such as PEG, and is a A1-B-A2 triblock copolymer having a number average molecular weight of 8000 to 500000. The biocompatible material of the present invention contains the triblock copolymer as a main component, and may be used as a tissue anti-adhesion barrier.
Owner:NOF CORP
Depsipeptide compound, and preparation method and application thereof
ActiveCN104804071AHigh insecticidal activitySimple production processBiocideMicroorganism based processesChemical structureMicroorganism
The invention relates to a microbe-derived pesticide, and concretely relates to a depsipeptide compound, and a preparation method and an application thereof. The depsipeptide compound is represented by formula (1). The depsipeptide compound is obtained by through fermenting culture of Beauveria felina AS-70 (preserved in China General Microbiological Culture Collection Center on Sep. 29, 2012 with the preservation number of CGMCC NO.6643), and through extraction and separation. A result of identification of the chemical structure of the compound through nuclear magnetic resonance and mass spectrum shows that the compound has good insecticidal activity.
Owner:INST OF OCEANOLOGY - CHINESE ACAD OF SCI
Method of re-sensitizing vancomycin resistant bacteria which selectively cleave a cell wall depsipeptide
The present invention relates to pyrrolidine compounds of the general structure: where n is an integer 1-6 and R is hydrogen or a C1 to C6 straight chain or branched alkyl group, and wherein when n=1, R=CH3 or H, useful for re-sensitizing vancomycin resistant Gram-positive bacteria in which resistance results from the conversion of an amide bond to an ester bond on the cell wall peptide precursors of the bacteria.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Novel depsipeptide and uses thereof
Owner:NOVOBIOTIC PHARMA LLC
Cryptophycin-based antibody-drug conjugates with novel self-immolative linkers
InactiveUS20180078656A1Improve efficiencyImmunoglobulins against cell receptors/antigens/surface-determinantsAntiinfectivesDiketopiperazinesWilms' tumor
The present invention relates to antibody- or peptide-drug conjugate compounds where one or more cryptophycin derivatives (macrocyclic depsipeptide) are covalently attached by a self-immolative linker which binds to one or more tumor-associated antigens or cell-surface receptors. The linker contains a cleavage site for proteases and a dipeptide unit able to form a diketopiperazine. These compounds may be useful in methods of diagnosis or treatment of cancer, and other diseases and disorders, such as immune or infective diseases.
Owner:EXIRIS SRL
Temperature responsive depsipeptide polymer
There is provided a temperature responsive polymer compound which comprises a repeating unit represented by the following general formula (I):—R1-Hmb-R2— (I)where, Hmb represents a valic acid residue represented by the following formula (II); R1 represents an amino acid, a polypeptide, or a hydroxy acid being linked by ester bond; R2 represents an amino acid or a polypeptide being linked by amide bond or a hydroxy acid being linked by ester bond:and wherein said polymer compound has 18 or more of amino acids residues and hydroxy acid residues in total.
Owner:GUNMA UNIVERSITY
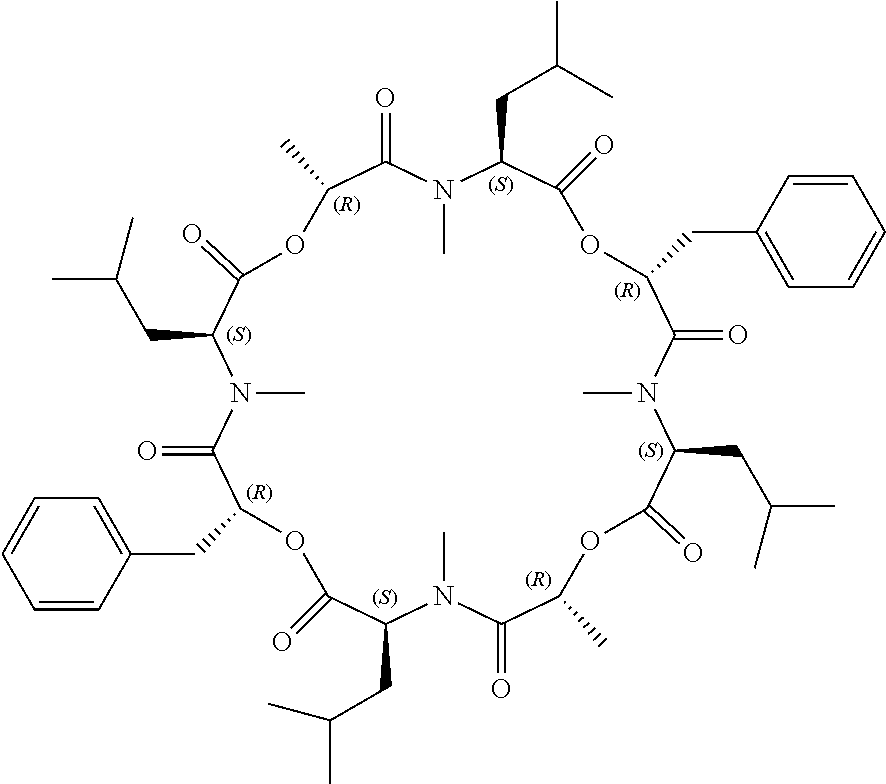
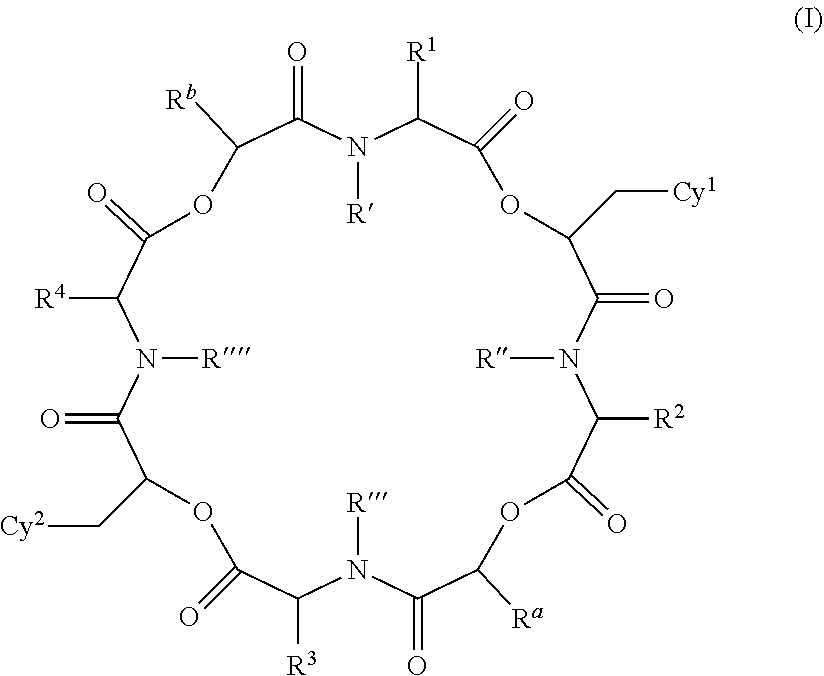
Anthelmintic depsipeptide compounds
The present invention provides cyclic depsipeptide compounds of formula (I) and compositions comprising the compounds that are effective against parasites that harm animals, including humans. The compounds and compositions may be used for combating parasites in or on animals including mammals and birds. The invention also provides for an improved method for eradicating, controlling and preventing parasite infestation in animals, including birds and mammals.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Injectable formulation of antibiotic and solution for intravenous administration thereof
InactiveUS7968588B2Remarkable antibacterial activityReduce the burden onAntibacterial agentsBiocideHigh concentrationAntibiotic Y
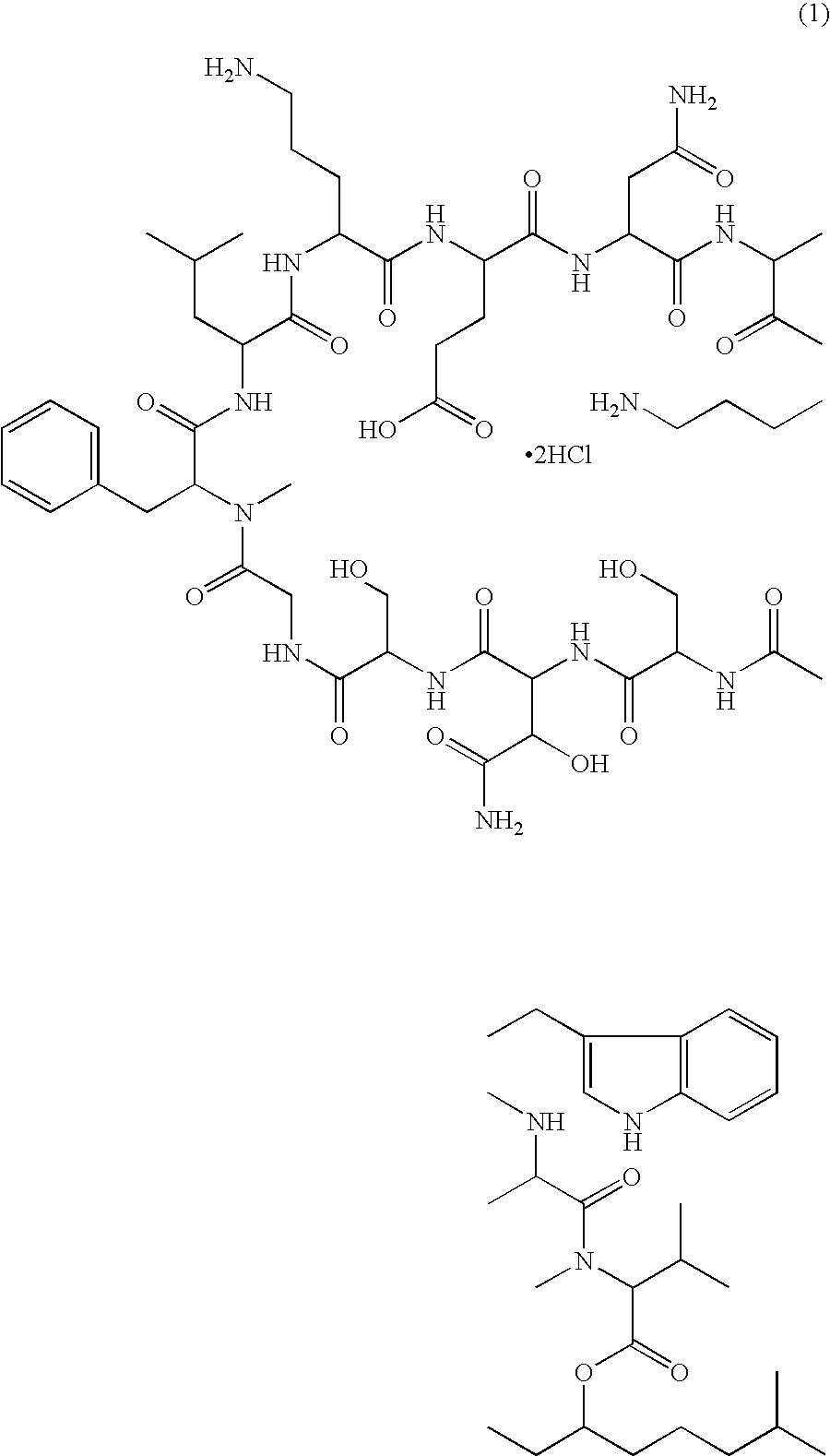
A pharmaceutical composition for injection comprising a depsipeptide antibiotic, WAP-8294A2, as an active ingredient, which is stable and contains WAP-8294A2 in high concentrations is provided. This composition comprises WAP-8294A2 of the following structural formula (1) as an active ingredient and is characterized in that 2-hydroxypropyl-β-cyclodextrin or β-cyclodextrin is contained as a stabilizer or solubilizer and the pH of the composition is not adjusted. This composition is mixed with a pH-adjusting agent such as dextrose and with an infusion or diluent comprising a solution of disodium hydrogen phosphate, sodium dihydrogen phosphate, and sodium hydroxide at the time of use to prepare a solution for intravenous administration of WAP-8294A2.
Owner:KYOTO BIOPHARMA INC
Cyclic depsipeptide compounds and their uses
InactiveUS20150259386A1Reduced immunosuppressive activityHigh affinityAntimycoticsNervous disorderDiseaseSolubility
The present invention relates to novel cycloundecadepsipeptide compounds and their analogues which bind and inhibit cyclophilins, have reduced immunosuppressive activity and improved physicochemical properties including water solubility. The present invention further relates to pharmaceutical compositions containing said depsipeptide compounds and their analogues for use in the treatment or prevention of diseases and pathologies which may be ameliorated by the inhibition of cyclophilin activity.
Owner:CYPRALIS
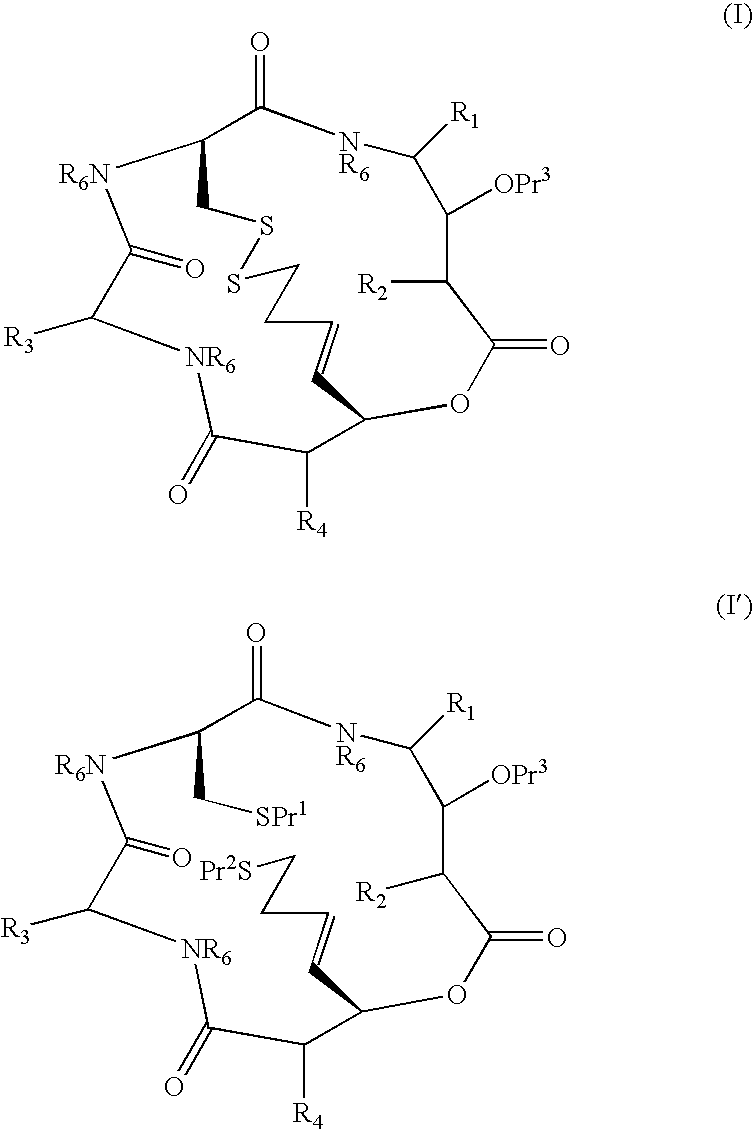
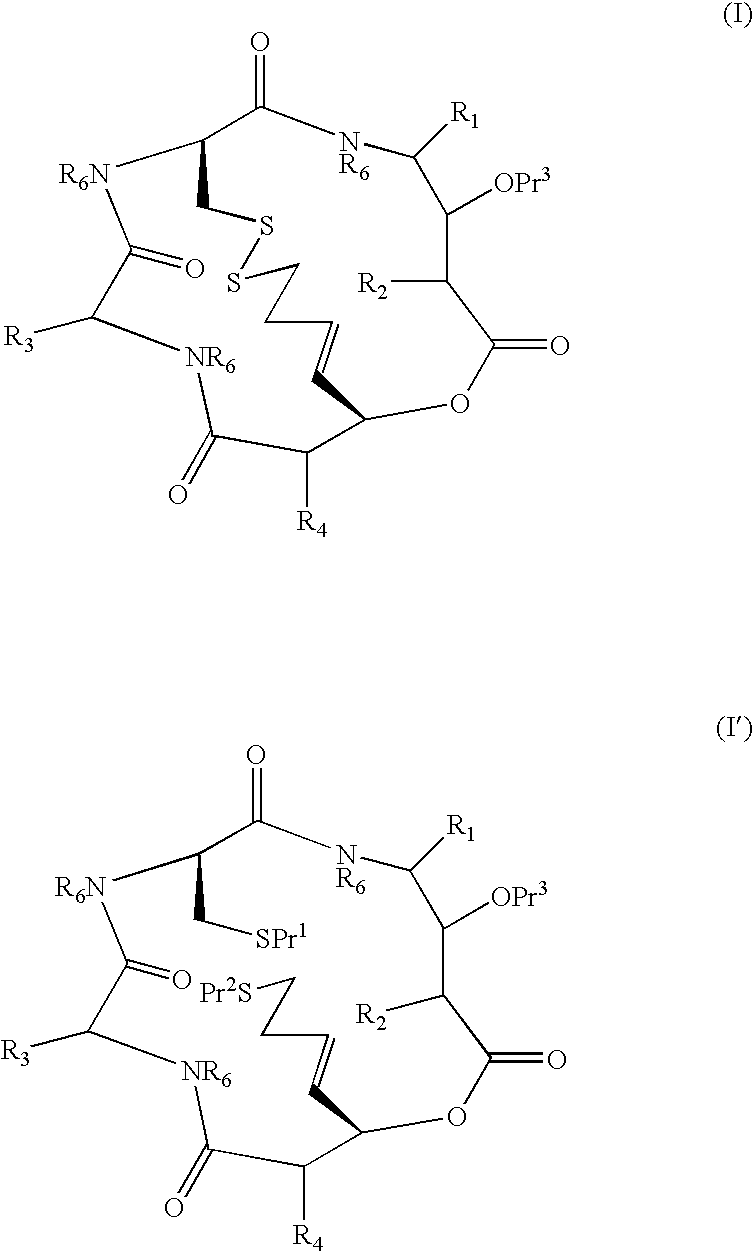
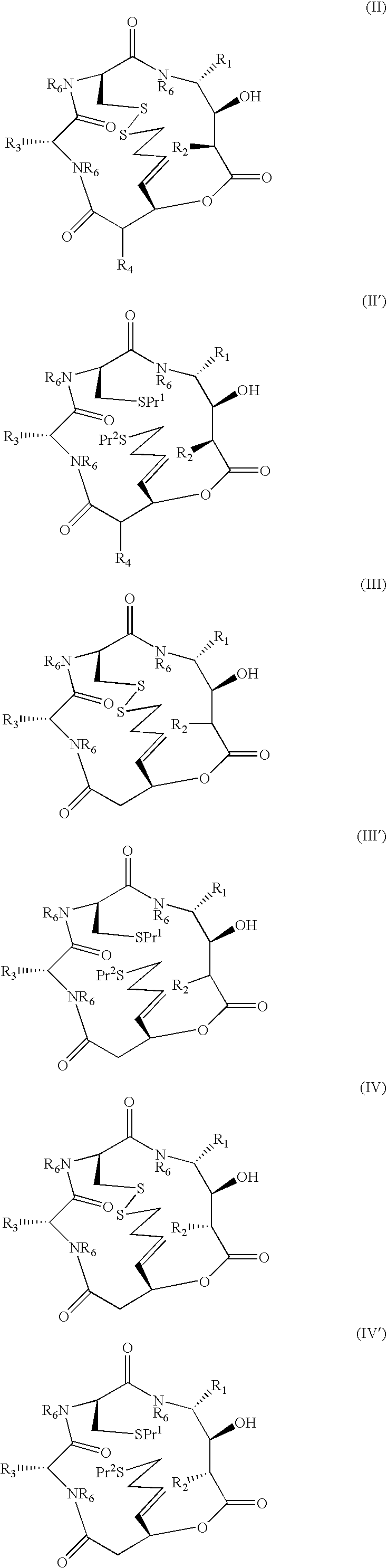
Depsipeptides and their therapeutic use
Compounds which are Spiruchostatin analogues of the general formula (I) or (I′), isosteres thereof and pharmaceutically acceptable salts thereof are found to inhibit HDAC wherein R1, R2, R3 and R4 are the same or different and represent an amino acid side chain moiety and each R6 is the same or different and represents hydrogen or C1-C4 alkyl.
Owner:UNIV OF SOUTHAMPTON
Crystal of depsipeptide derivative and process for producing the same
InactiveUS6346603B1Increase productivityHigh crystal yieldDepsipeptidesImmunoglobulinsFiltrationSpecific volume
Crystals of a depsipeptide derivative of formula (IV):having an antiparasitic activity and methods for producing such crystals. Such crystals include crystals (I), (II) and (III) that have particular physicochemical properties or characteristics, such as excellent filtration properties during crystallization, great fluidity, good specific volume. As a consequence, such crystals have improved handling ability during production resulting in enhanced production efficiency and increased crystal yield. Methods are also provided for producing such crystals.
Owner:ASTELLAS PHARMA INC
Butelase-mediated peptide ligation
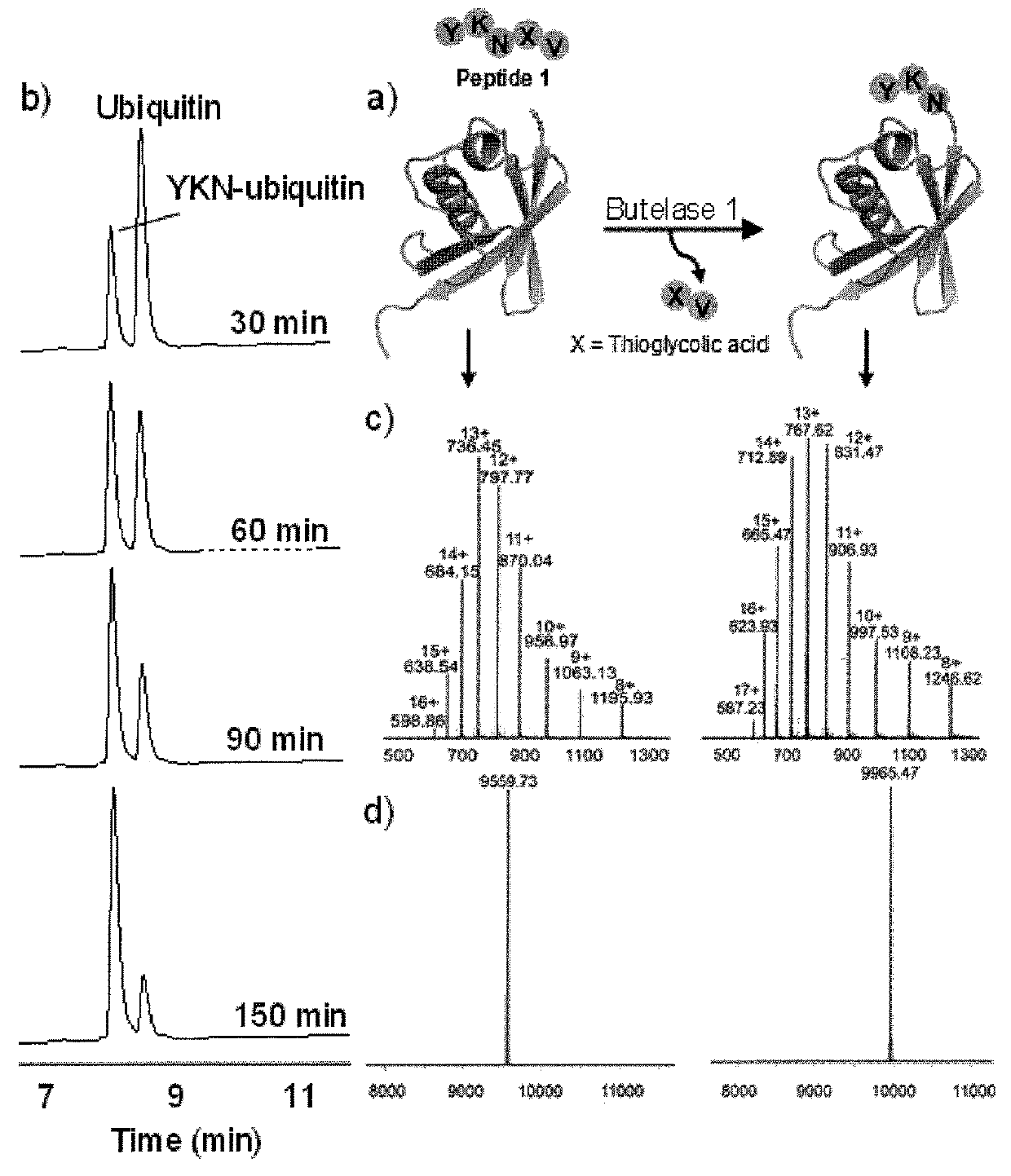
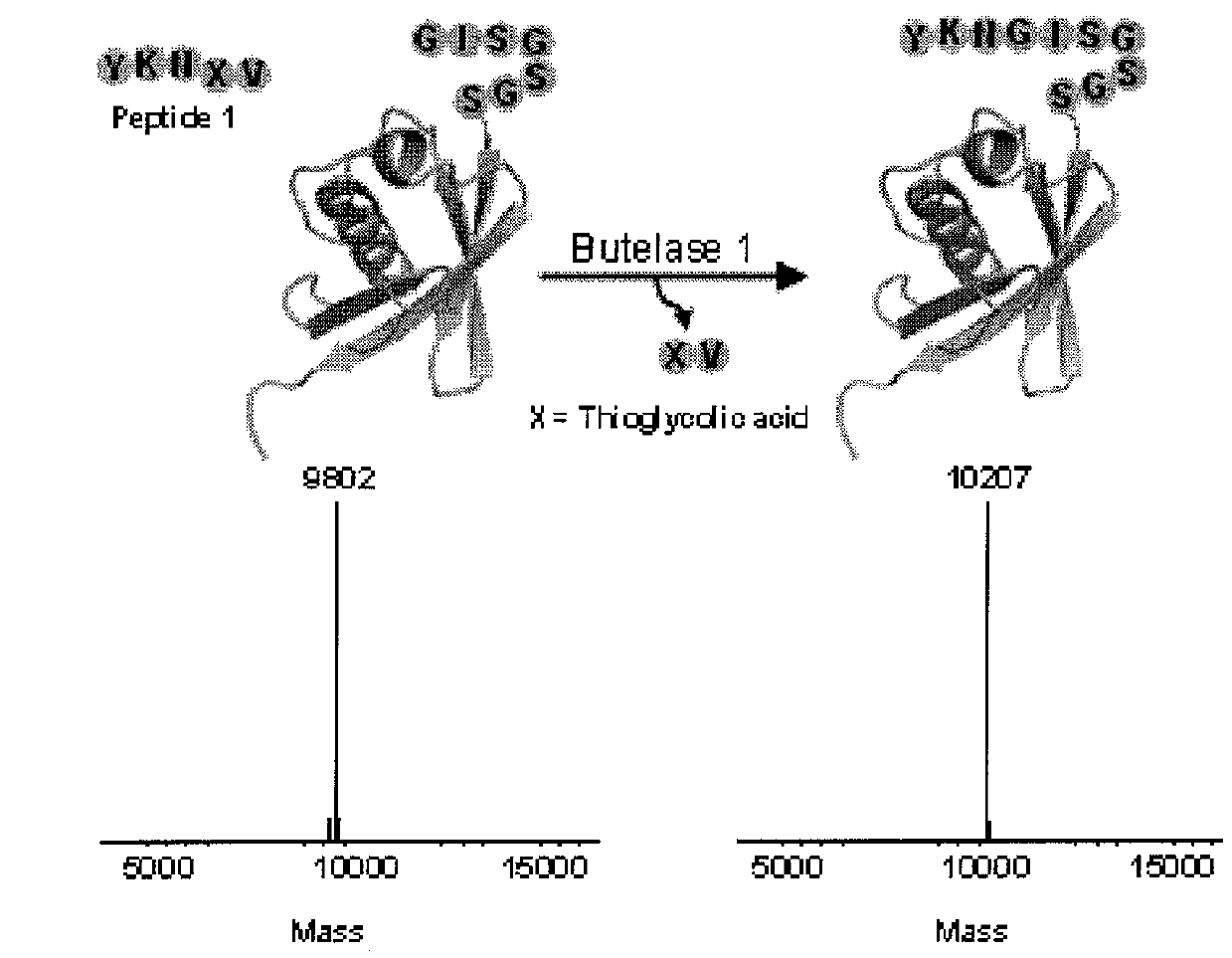
The present invention relates to a method of forming a peptide of Formula (I) (P1-Xaa1-Xaa2-P2) by ligating a first peptide of Formula (II) (P1-Xaa1-X—R, wherein X is O or S) to a second peptide of Formula (II I) (Xaa1-Xaa2-P2) by enzymatically cleaving the bond between “Asx” and “X” in the first peptide of Formula (II) and ligating the fragment P1-Asx of the first peptide to the second peptide of Formula (III), wherein the enzymatic cleavage and ligation reaction is catalyzed by butelase 1 (SEQ ID NO: 1) and the peptide of Formula (I) is a depsipeptide, preferably a thiodepsipeptide. Further encompassed are peptides and dendrimeric peptide assemblies prepared using the presently disclosed method, as well as use of the dendrimeric peptide assemblies as a vaccine, medicament, or diagnostic agent, particularly as an antimicrobial agent.
Owner:NANYANG TECH UNIV
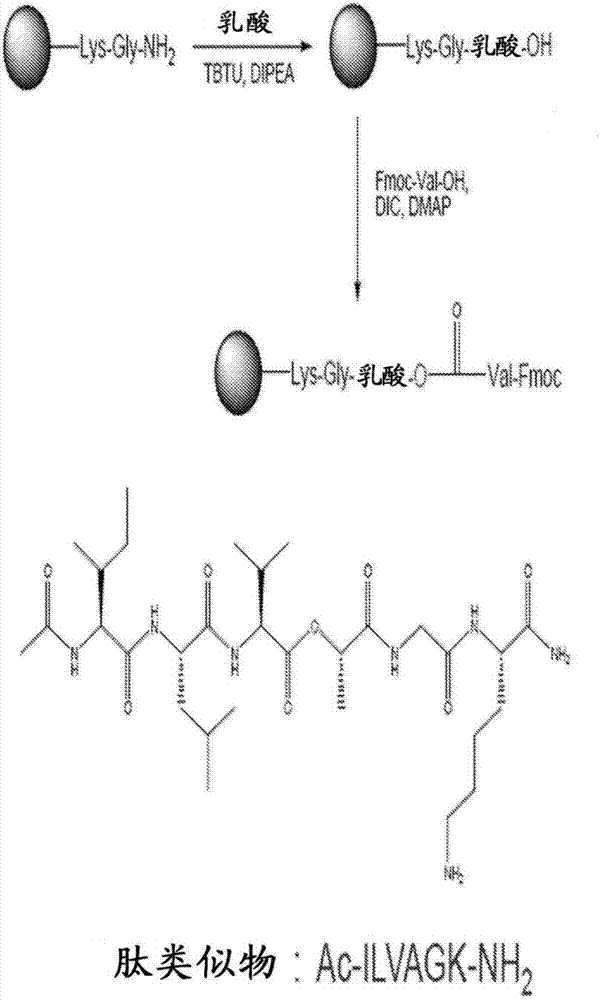
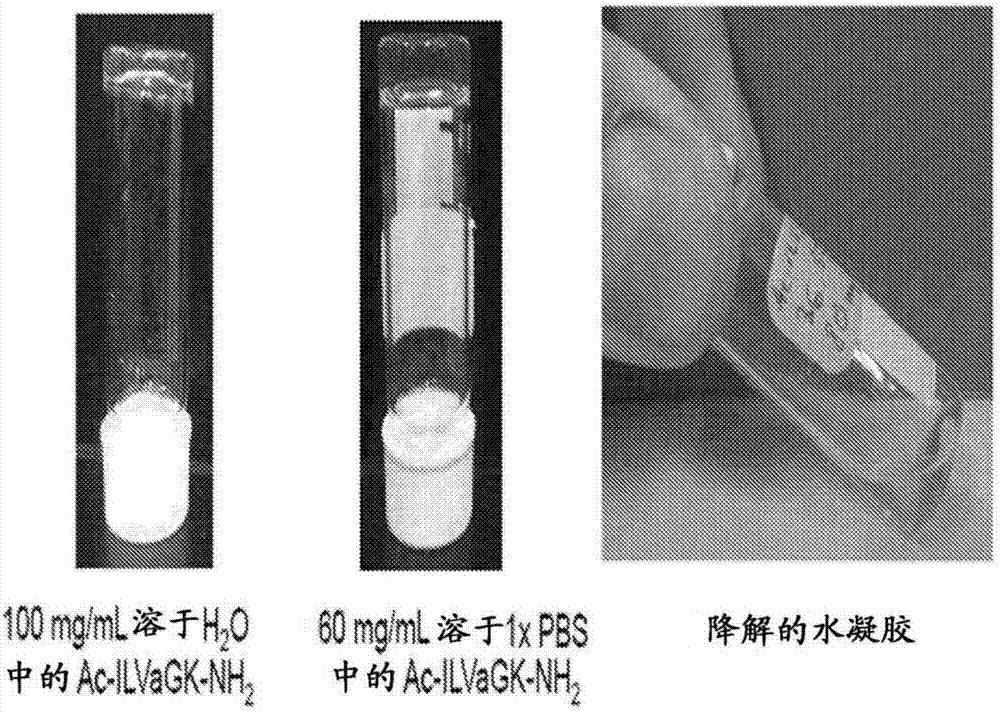
Self-assembling ultrashort aliphatic depsipeptides for biomedical applications
InactiveCN107406487ASkeletal disorderPharmaceutical delivery mechanismTissue replacementDepsipeptide
The present invention relates to ultrashort depsipeptides which are capable of self-assembling into hydrogels. One preferred embodiment is of Ac-ILVaGK-NH2, where a represents lactic acid. The invention also relates to the use of these depsipeptides in formulating hydrogels, co-gels or co-hydrogels, and pharmaceutical compositions or biomedical device or surgical implants or kits comprising these depsipeptides for various therapeutic applications such as regenerative medicine, tissue regeneration and tissue replacement.
Owner:AGENCY FOR SCI TECH & RES
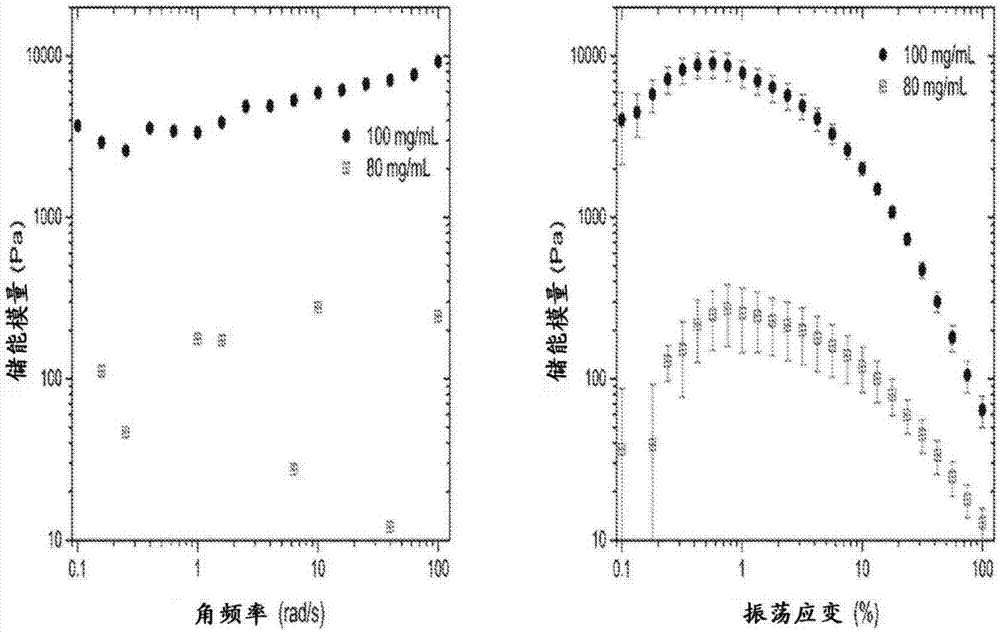
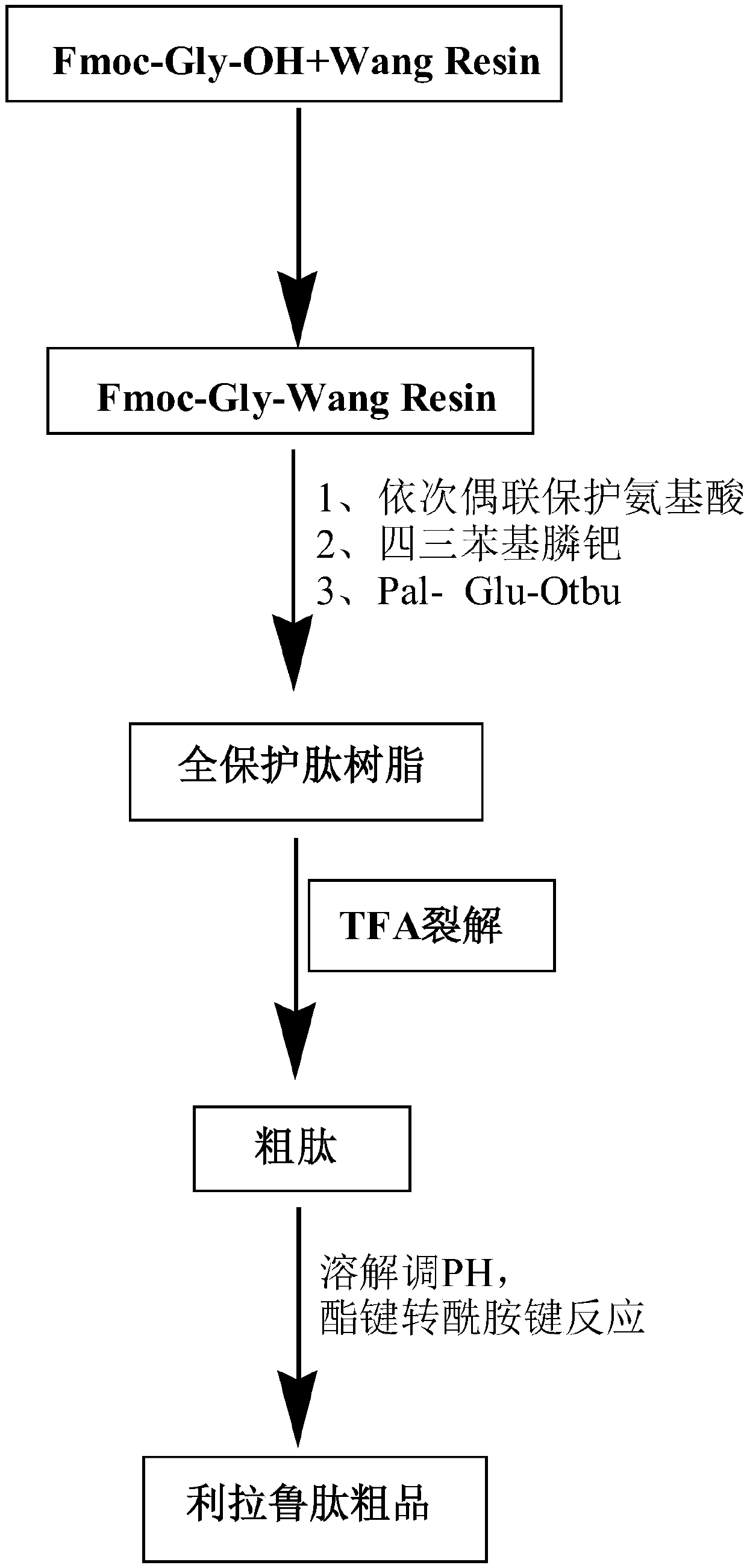
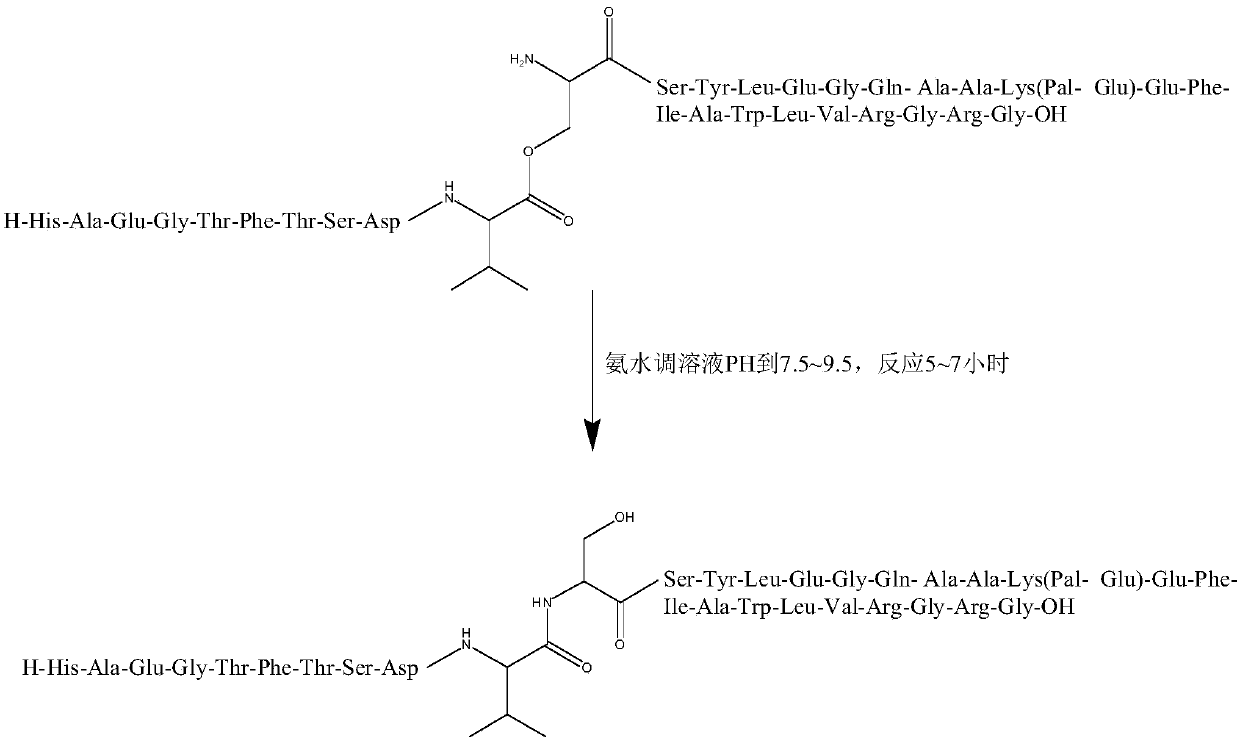
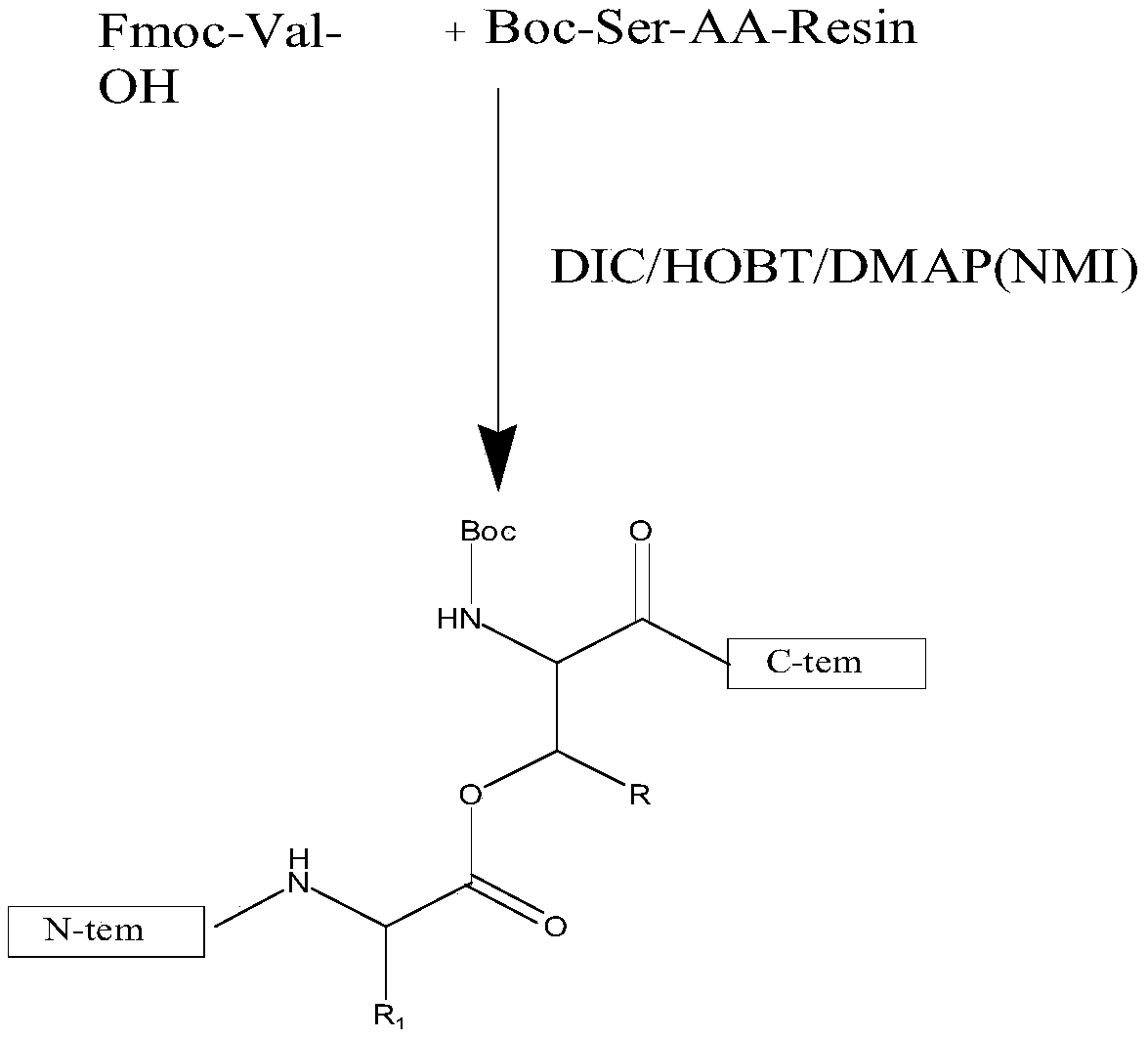
Liraglutide synthesis method
ActiveCN108676086ATroubleshoot compositingHigh purityPeptide preparation methodsBulk chemical productionSynthesis methodsWang resin
The invention discloses a liraglutide synthesis method, which comprises the following steps: 1, preparing a Fmoc-Gly-Wang resin; 2, preparing a fully-protected peptide resin from the Fmoc-Gly-Wang resin, protected amino acid and Pal-Glu, the fully-protected peptide resin comprising Depsipeptide Units; 3, performing TFA pyrolysis on the peptide resin to obtain crude peptide; 4, dissolving the crudepeptide obtained in Step 3, regulating the PH and performing ester bond to amido bond reaction to obtain a liraglutide crude product. According to the method, the Depsipeptide Units are introduced toobtain the fully-protected peptide resin and then pyrolysis and ester bond to amido bond reaction are performed to obtain a target product; by introduction of the Depsipeptide Units, problems about synthesis of difficult sequences of the liraglutide can be solved, and the purity and the yield of the synthesized crude peptide can be greatly improved.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD +1
Injectable formulation of antibiotic and solution for intravenous administration thereof
InactiveUS20090258920A1Increase concentrationOvercome disadvantagesAntibacterial agentsBiocideHigh concentrationAntibiotic Y
A pharmaceutical composition for injection comprising a depsipeptide antibiotic, WAP-8294A2, as an active ingredient, which is stable and contains WAP-8294A2 in high concentrations is provided. This composition comprises WAP-8294A2 of the following structural formula (1) as an active ingredient and is characterized in that 2-hydroxypropyl-β-cyclodextrin or β-cyclodextrin is contained as a stabilizer or solubilizer and the pH of the composition is not adjusted. This composition is mixed with a pH-adjusting agent such as dextrose and with an infusion or diluent comprising a solution of disodium hydrogen phosphate, sodium dihydrogen phosphate, and sodium hydroxide at the time of use to prepare a solution for intravenous administration of WAP-8294A2.
Owner:KYOTO BIOPHARMA INC
Novel Aldehyde Acetal Based Processes for the Manufacture of Macrocyclic Depsipeptides and New Intermediates
The invention relates to process for the chemical manufacture of depsipeptides of the formula (I) employing an aldehyde acetal intermediate, (Formula I) wherein the symbols have the meaning defined in the description, to new intermediates and their manufacture, as well as related invention embodiments.
Owner:NOVARTIS AG
Anthelmintic depsipeptide compounds
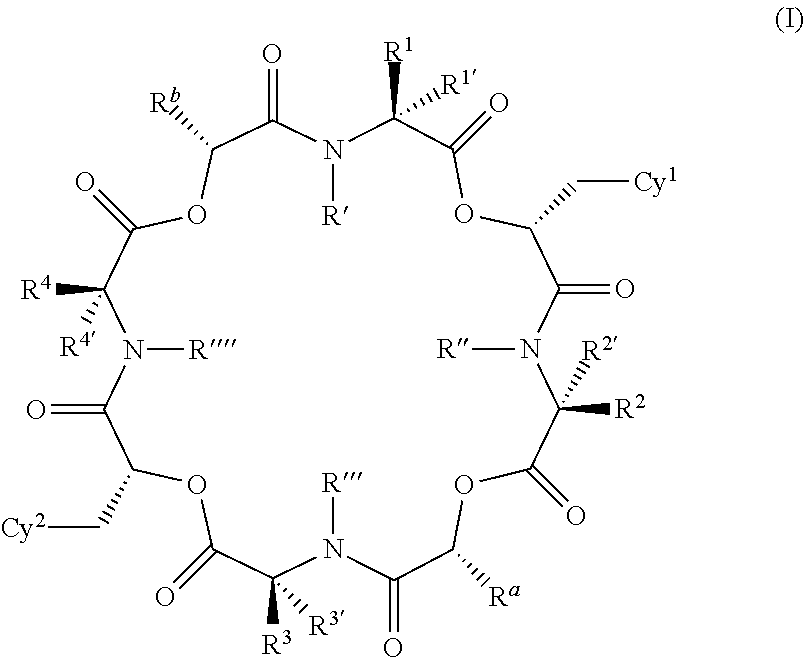
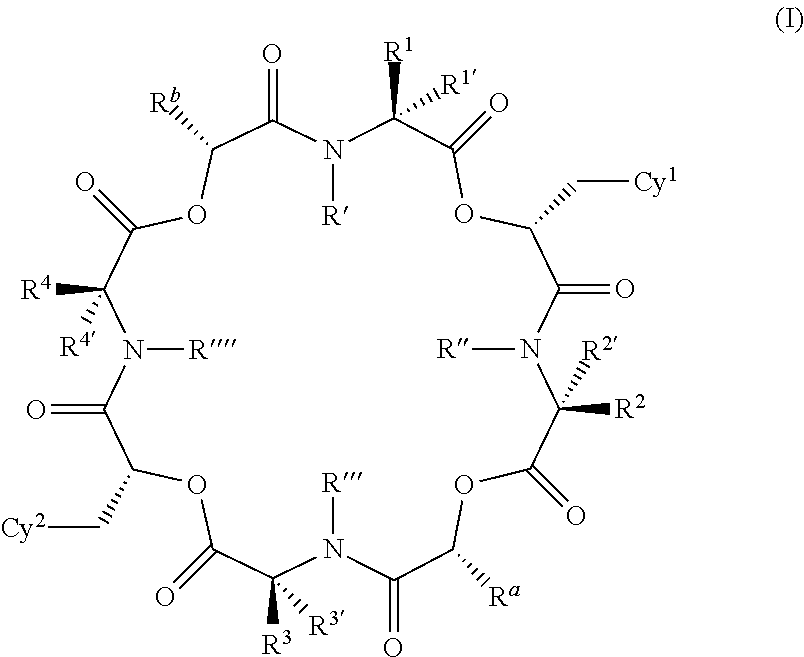
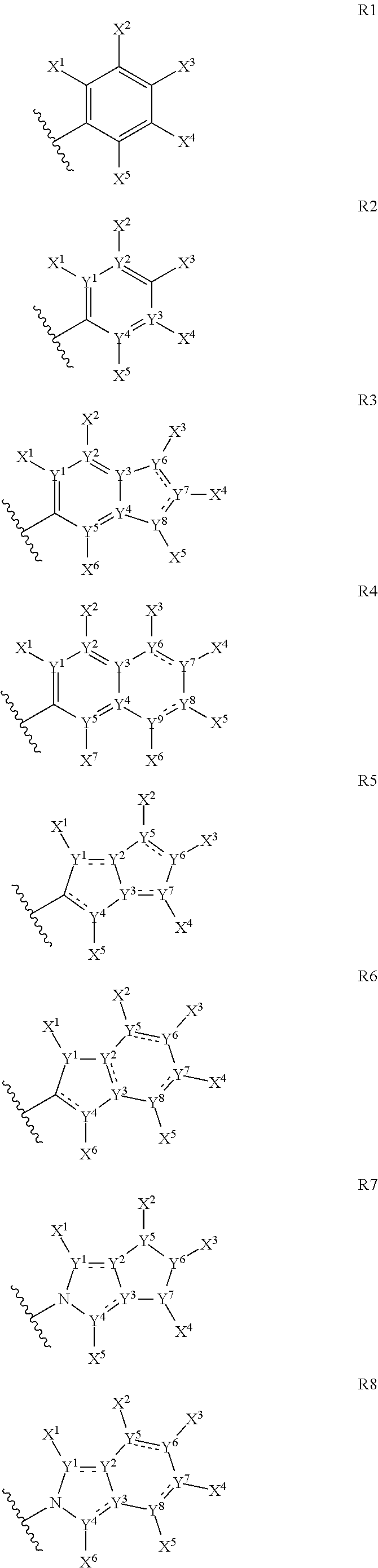
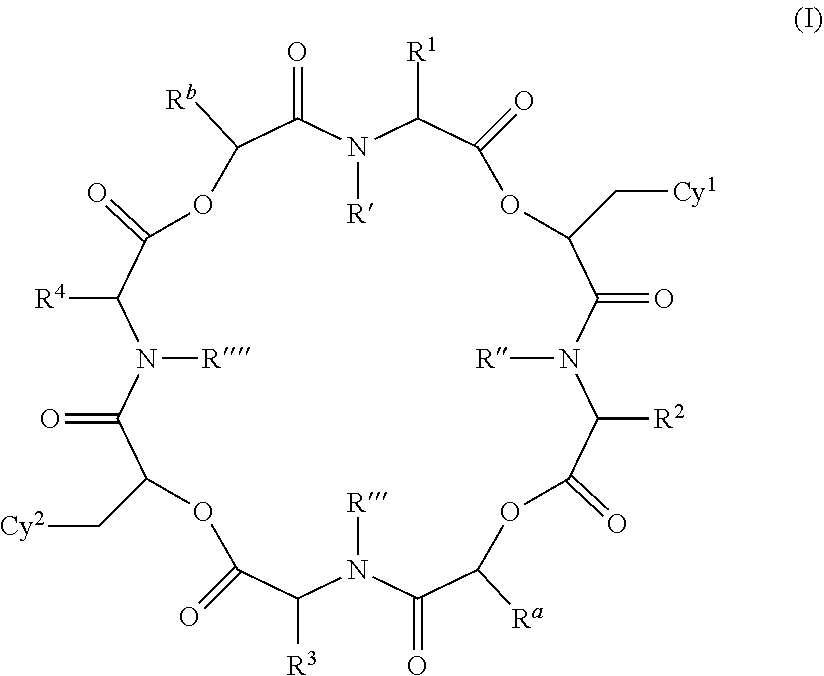
The present invention provides cyclic depsipeptide compounds of formula (I) wherein the stereochemical configuration of at least one carbon atom bearing the groups Cy1, Cy2, R1, R2, R3, R4, Ra and Rb is inverted compared with the naturally occurring cyclic depsipeptide PF1022A. The invention also provides compositions comprising the compounds that are effective against parasites that harm animals. The compounds and compositions may be used for combating parasites in or on mammals and birds. The invention also provides for an improved method for eradicating, controlling and preventing parasite infestation in birds and mammals.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com