Method for synthesizing dioxalate group lithium borate
A technology of lithium oxalate borate and synthesis method is applied in chemical instruments and methods, compounds containing elements of Group 3/13 of the periodic table, organic chemistry, etc. Simple process, improved high temperature cycle performance, less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0020] Example 1 LiB(C 2 o 4 ) 2 Synthesis
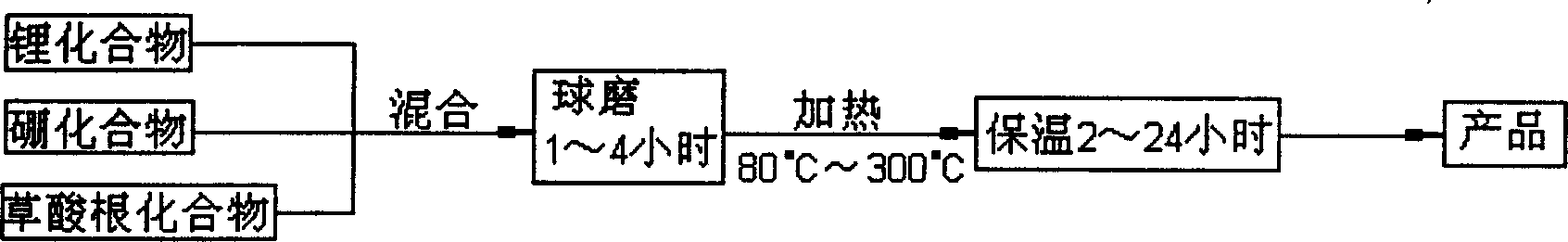
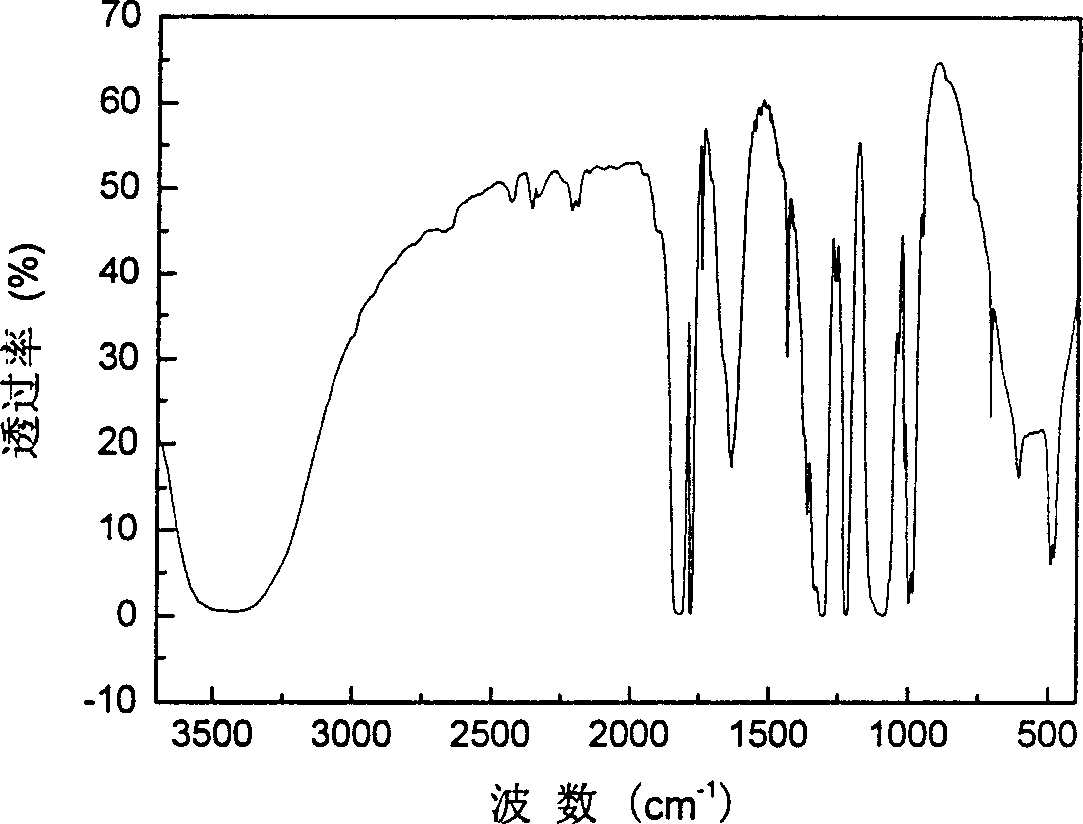
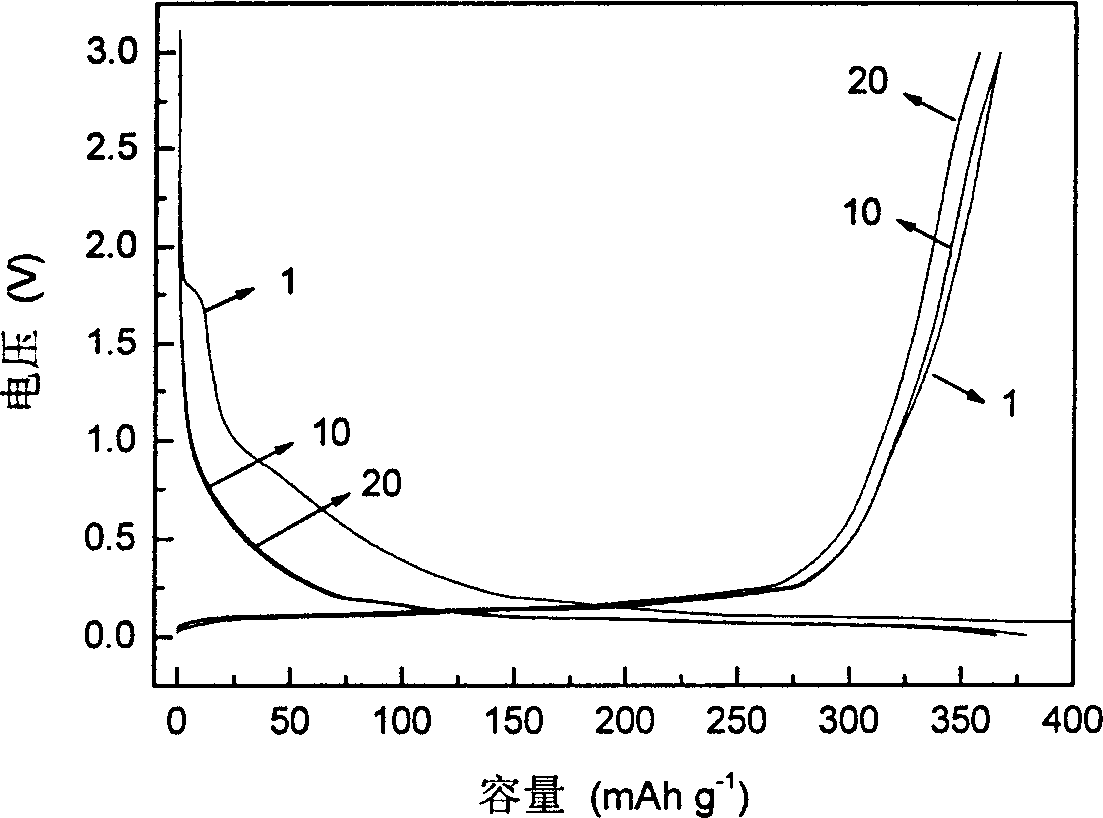
[0021] Take 151.31 grams of oxalic acid, 45.06 grams of lithium carbonate, and 37.08 grams of boric acid, mix and ball mill them at 5°C for 2 hours, and then place them under the protection of argon at 300°C for 2 hours to obtain LiB(C 2 o 4 ) 2 111.6 g, 96% yield. The specific synthesis process is as figure 1 shown. The molecular structure of the resulting product was determined by infrared spectroscopy, as figure 2 shown. The resulting product is formulated into 0.8mol L -1 LiB(C 2 o 4 ) 2 EC / EMC / DEC electrolytes are applied to Li / graphite batteries for charge and discharge tests. The battery charge and discharge cut-off voltage is 3.000V~0.005V, and the current density is 33mA·g - 1 , The test temperature is 55°C. Test results such as image 3 shown. After charging and discharging tests, it can be found that using LiB(C 2 o 4 ) 2The Li / graphite battery with electrolyte has high charge-discharge capacity and ...
example 2
[0022] Example 2 LiB(C 2 o 4 ) 2 Synthesis
[0023] Take 113.46 grams of oxalic acid, 18.88 grams of lithium hydroxide, and 27.81 grams of boric acid, mix and ball mill them at 70°C for 4 hours, and then place them under nitrogen protection at 110°C for 6 hours to obtain LiB(C 2 o 4 ) 2 85.96 g, 98.6% yield. The resulting product is formulated into 0.8mol L -1 LiB(C 2 o 4 ) 2 / PC electrolyte was applied to Li / graphite battery for charge and discharge test. The battery charge and discharge cut-off voltage is 1.700V ~ 0.005V, and the current density is 33mA g -1 , the test temperature is 25°C. Test results such as Figure 4 shown. After charging and discharging tests, it can be found that LiB(C 2 o 4 ) 2 The use of electrolyte can effectively inhibit the structural damage of PC to graphite materials during charge and discharge, thus making it possible to use PC components in lithium-ion batteries.
example 3
[0024] Example 3 LiB(C 2 o 4 ) 2 Synthesis
[0025] Take 113.99 grams of lithium hydrogen oxalate, 126.07 grams of oxalic acid, and 43.83 grams of metaboric acid, mix and ball mill them at 25°C for 1 hour, and then place them under vacuum protection at 80°C for 24 hours to obtain LiB(C 2 o 4 ) 2 189.2 g, 97.7% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com