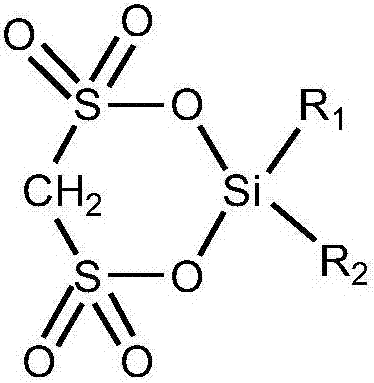
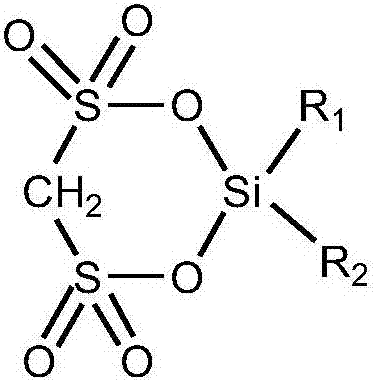
Cyclic silyl disulfonate and preparation method thereof
A technology of silyl disulfonate and methylene disulfonic acid, applied in secondary batteries, organic silicon compounds, electrochemical generators, etc. The effect of strong practicability, high product purity and broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The preparation method of above-mentioned cyclic disulfonic acid silicon base ester, comprises the steps:
[0038] With methylene disulfonic acid or the methylene disulfonate of general formula (I) and the dihydrocarbyl diactive functional group silane of general formula (II) in solvent, according to methylene disulfonic acid or methylene disulfonic acid The molar ratio of sulfonate and dihydrocarbyl diactive functional group silane to solvent is 1:0.1~10:3~50 to react;
[0039] Or the methylene disulfonic acid or the methylene disulfonate of the general formula (I) and the dihydrocarbyl cyclosiloxane of the general formula (III) in a solvent, according to the methylene disulfonic acid or the methylene disulfonic acid The molar ratio of disulfonate, dihydrocarbyl cyclosiloxane and solvent is 1:0.1~5:3~50 to react;
[0040] Or the dihydrocarbyl ring silazane of methylene disulfonic acid or general formula (I) and general formula (IV) according to methylene disulfonic ac...
Embodiment 1
[0063] Add 1mol sodium methanedisulfonate into a three-neck flask equipped with a thermometer, magnetic stirring, reflux condenser, and constant pressure dropping funnel, heat in an oil bath at about 100°C, and dehydrate under negative pressure for 2 hours under a slight nitrogen flow. Cool down to room temperature, remove the vacuum with a nitrogen bag, quickly add solvents that have been dehydrated to below 5 ppm: 1 mol of fluorobenzene, 1 mol of dichloroethane and 1 mol of dimethylformamide, and dropwise add 1 mol of dimethyldichlorosilane while stirring, After the dropwise addition, continue to stir for 1 hour, then heat to 80-85°C and reflux for 12 hours, cool to about 10°C, press filter, transfer the filtrate to a rotary evaporator to evaporate dichloroethane and dimethyl Formamide, through repeated recrystallization purification and GC-MS analysis, the structural formula is The target product 98g, purity 99.6%.
Embodiment 2
[0065] Add 1 mol of silver methanedisulfonate into a three-neck flask equipped with a thermometer, magnetic stirring, reflux condenser, and constant pressure dropping funnel, heat in an oil bath at about 100°C, and dehydrate under negative pressure for 2 hours under a slight nitrogen flow. Cool down to room temperature, use a nitrogen bag to eliminate the vacuum, quickly add 50 mol of dioxane that has been dehydrated to less than 5 ppm of moisture, and add 5 mol of methylvinyldibromosilane dropwise while stirring. After the dropwise addition, continue to stir for 1 hour, and then heat Reflux at 100-105°C for 25 hours, cool to about 5°C, filter with suction, transfer the filtrate to a rotary evaporator to evaporate dioxane and excess methylvinyldibromosilane under negative pressure, and recrystallize several times Purification and GC-MS analysis, the structural formula is The target product 116g, purity 99.3%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com