Synthetic method of polypeptide thymosin alpha1
A thymic method, solid-phase synthesis technology, applied in the preparation methods of peptides, chemical instruments and methods, peptides, etc., can solve the problems of low final yield and insufficient peptide purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
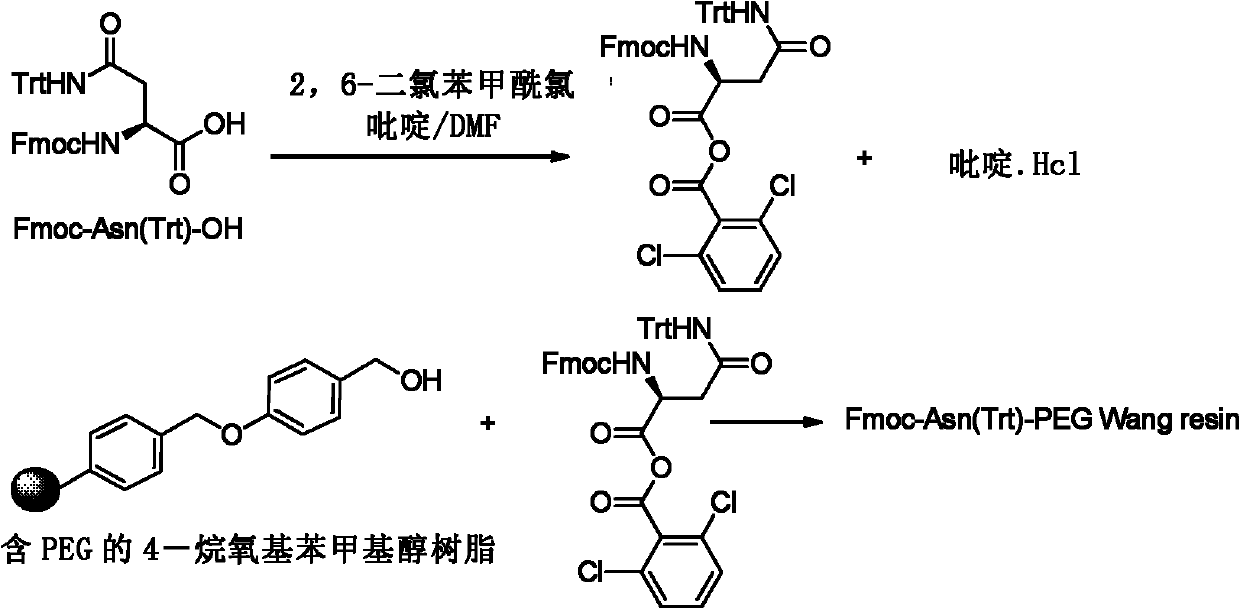
[0033] In an example of the present invention, the new preparation method of the polypeptide solid-phase synthesis thymus method of the present invention includes the following steps:
[0034] In the first step, Fmoc-Asn(Trt)-OH and resin are bonded to obtain Fmoc-Asn(Trt)-resin; preferably using hydroxyl functional resin, more preferably PEG-Wang resin, the substitution rate of the PEG-Wang resin 0.2-0.8mmol / g;
[0035] In the second step, the deprotecting agent of the present invention is mixed with the Fmoc-Asn(Trt)-resin, and the Fmoc group is removed to obtain the Asn(Trt)-resin;
[0036] In the third step, Fmoc-Glu(OtBu)-OH and Asn(Trt)-resin are condensed to obtain Fmoc-Glu(OtBu)-Asn(Trt)-resin;
[0037] The fourth step, using the deprotecting agent of the present invention to remove the Fmoc group;
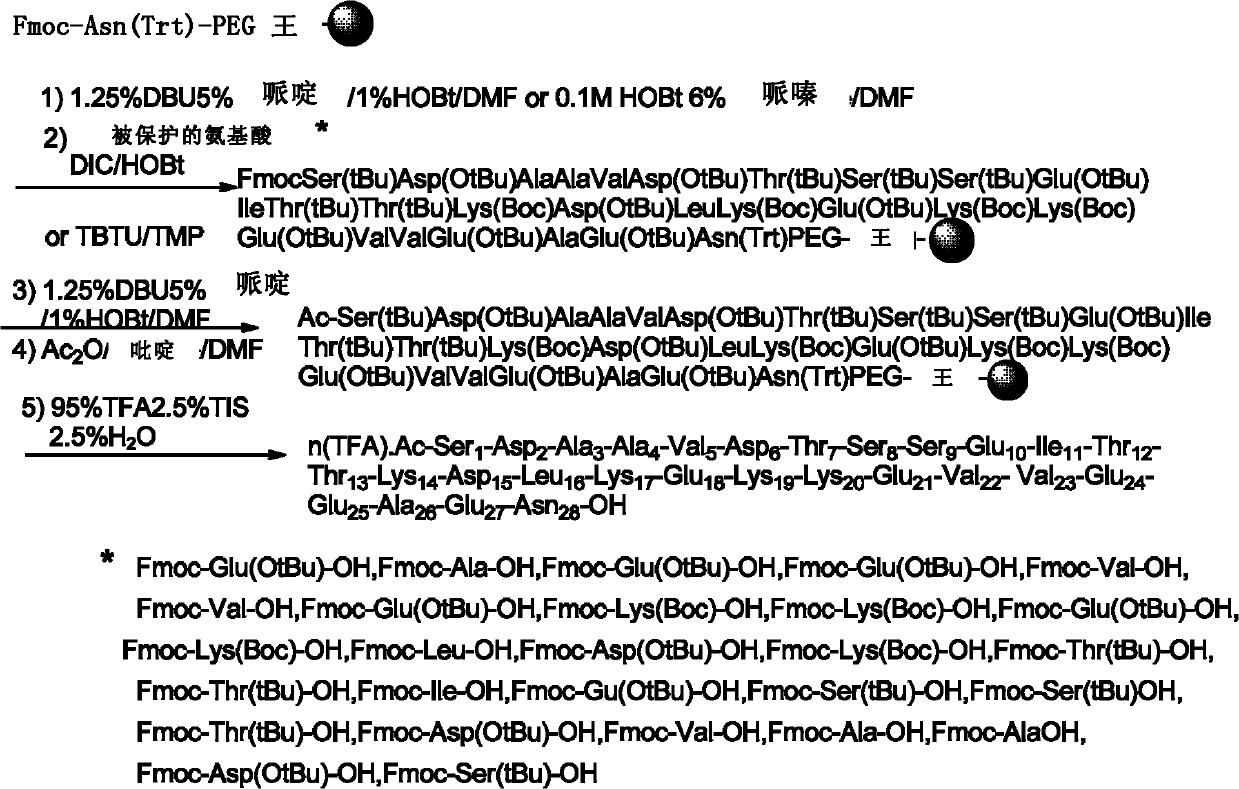
[0038] The 5th step, repeat above-mentioned peptide bond formation step, make peptide chain grow from C terminal to N terminal, until obtaining FmocSer(tBu)Asp(OtBu)AlaA...
Embodiment 1
[0061] Preparation of Fmoc-Asn(Trt)-PEG-King Resin
[0062] Soak 15g of PEG-King resin (substitution degree is 0.5-0.7mmol / g) in 150mlDMF for 30 minutes, drain, and 3.0 equivalents (19.33g, 32.4mmoles) of Fmoc-Asn(Trt)-OH, 4.8 equivalents (7.41 mL, 51.8mmol) of 2,6-dichlorobenzoylchloride and 8.4 equivalents (7.4mL, 90.7mmol) of DMF solution were mixed for esterification reaction for 3 hours. Then the resin was washed with DMF, and the resin was capped with 100 mL of 10% pyridine / DMF and 100 mL of 10% benzoyl chloride (benzoyl chloride) / DMF mixture for 1 hour. The resin was washed with DMF and methanol, dried in vacuum, and the degree of substitution was measured (the degree of substitution was 0.43mmol / g). See figure 1 .
[0063] Fmoc deprotection
[0064] Use 0.2MHOBt / 6%piperazine / DMF to remove Fmoc, and deprotect twice consecutively, the time is 10min and 20min respectively. Drain the deprotection solution and wash with DMF and methanol respectively. After exhaustion,...
Embodiment 2
[0080] Preparation of Fmoc-Asn(Trt)-PEG-King Resin
[0081] Soak 15g of PEG-King resin (substitution degree is 0.5-0.75mmol / g) in 150mlDMF for 30 minutes, drain, and 3.0 equivalents (19.33g, 32.4mmole) of Fmoc-Asn(Trt)-OH, 4.8 equivalents (7.41 mL, 51.8mmol) of 2,6-dichlorobenzoylchloride and 8.4 equivalents (7.4mL, 90.7mmol) of DMF solution were mixed for esterification reaction for 3 hours. Then the resin was washed with DMF, and capped with 100 mL of 10% pyridine / DMF and 100 mL of 10% benzoyl chloride / DMF mixture for 1 hour. The resin was washed with DMF and methanol, dried in vacuum, and the degree of substitution was measured.
[0082] Fmoc deprotection
[0083] Use 0.1MHOBt / 10%piperazine / DMF to remove Fmoc, and deprotect twice consecutively, the time is 10min and 20min respectively. Drain the deprotection solution and wash with DMF and methanol respectively. After exhaustion, Kaiser test was used to evaluate the removal of Fmoc.
[0084] Fmoc amino acid condensation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com