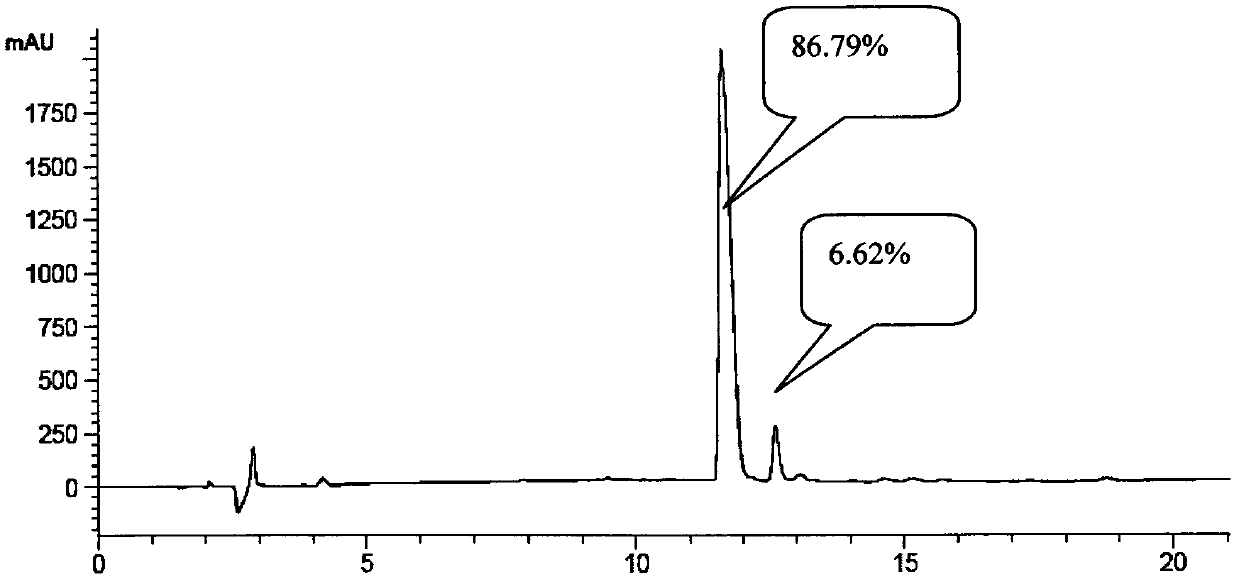
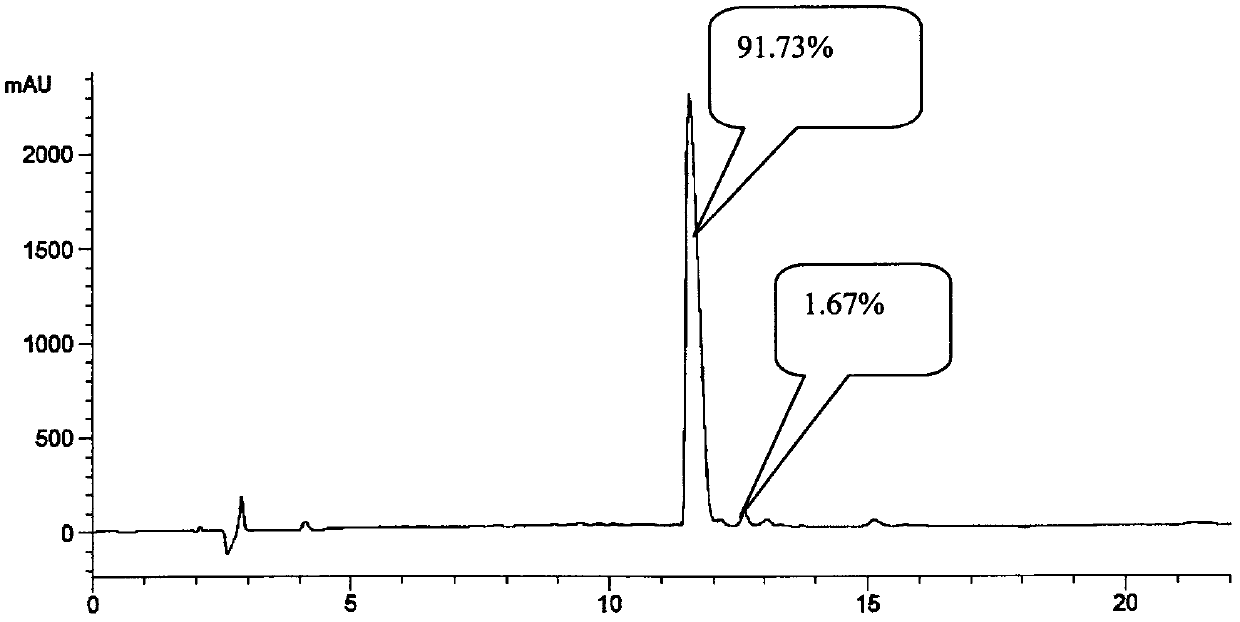
One-step method based solid-phase polypeptide synthesis method
A solid-phase synthesis and one-step technology, which is applied in the field of one-step solid-phase synthesis of peptides, can solve problems such as inconvenient operation, complicated preactivation process, and easy racemization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Example 1: Preparation of Fmoc-Phe-Wang resin
[0114] Weigh 50 g of Wang resin (100-200 mesh, 1.23 mmol / g), put it into a sand core reactor, wash once with 500 mL of DMF, drain, and then use 500 mL of DCM to fully swell the resin and drain. Add Fmoc-Phe-OH (MW: 387.4, 3 times the moles of Wang resin) 71.5g, HOBt (MW: 135.13, 3 times the moles of Wang resin) 24.9g, DIC (MW: 126.2, the moles of Wang resin) 3 times) 28.9mL, 300mL DMF, 100mL DCM, fully and uniformly contact the peptide resin with nitrogen flow, and use the volatilization endotherm of DCM to rapidly cool the entire reaction system to 16 ° C, slowly add DMAP (MW: 122, Wang resin moles) 0.2 times the number) 1.5 g, and the mixture was stirred at 26° C. for 18 hours. Vacuum dry, wash twice with DMF, and vacuum dry. Add Pyridine (MW: 79.1, 10 times the mole number of Wang resin) 49mL, Ac 2 O (MW: 102.09, 10 times the mole number of Wang resin) 58 mL, 500 mL DCM, the mixture was stirred at 27°C for 3 hours, d...
Embodiment 2
[0115] Example 2: Preparation of Fmoc-Pro-Phe-Wang resin
[0116] Add 500 mL of 20% Pip / DMF solution, stir at 25 °C for 10 minutes, vacuum dry, add 500 mL of 20% Pip / DMF solution, stir at 25 °C for 20 minutes, vacuum dry, wash once with DMF and twice with MeOH, DMF was washed once, DCM once, and dried in vacuo. Add Fmoc-Pro-OH (MW: 337.4, twice the moles of Fmoc-Phe-Wang resin) 40.5g, HOBt (MW: 135.13, twice the moles of Fmoc-Phe-Wang resin) 16.2g, HBTU (MW : 379.25, twice the moles of Fmoc-Phe-Wang resin) 45.5g, 350mL DMF, 100mL DCM, fully and uniformly contact the peptide resin with nitrogen flow, and use the volatilization endotherm of DCM to quickly cool the entire reaction system to 15 ℃ , 31.4 mL of DIPEA (MW: 129.24, twice the moles of Fmoc-Phe-Wang resin) was added dropwise, and the mixture was stirred at 25°C for 2.5 hours. Wash twice and vacuum dry.
Embodiment 3
[0117] Example 3: Preparation of Fmoc-His(Trt)-Pro-Phe-Wang resin
[0118] Add 500 mL of 20% Pip / DMF solution, stir at 25 °C for 10 minutes, vacuum dry, add 500 mL of 20% Pip / DMF solution, stir at 25 °C for 20 minutes, vacuum dry, wash once with DMF and twice with MeOH, DMF was washed once, DCM once, and dried in vacuo. Add Fmoc-His(Trt)-OH (MW: 619.7, twice the moles of Fmoc-Phe-Wang resin) 74.4g, HOBt (MW: 135.13, twice the moles of Fmoc-Phe-Wang resin) 16.2g, HBTU (MW: 379.25, twice the moles of Fmoc-Phe-Wang resin) 45.5g, 350mL DMF, 100mL DCM, fully and uniformly contact the peptide resin with nitrogen flow, and use the volatilization endotherm of DCM to rapidly cool down the entire reaction system To 18°C, 31.4 mL of DIPEA (MW: 129.24, twice the mole number of Fmoc-Phe-Wang resin) was added dropwise, the mixture was stirred at 23°C for 2 hours, the ninhydrin test was negative, vacuum dried, and washed once with MeOH , washed twice with DMF, and vacuum-dried.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Potency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com