Preparation method and application of polyvinyl acetal-based gel polymer electrolyte
A polyvinyl acetal and gel polymer technology, which is applied in the manufacture of electrolyte batteries, non-aqueous electrolyte batteries, circuits, etc., can solve the problems of limited assembly methods of lithium-ion batteries, poor electrochemical stability of gel films, and mechanical properties. Low-level problems, to achieve the effect of complete reaction, good thermal stability, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
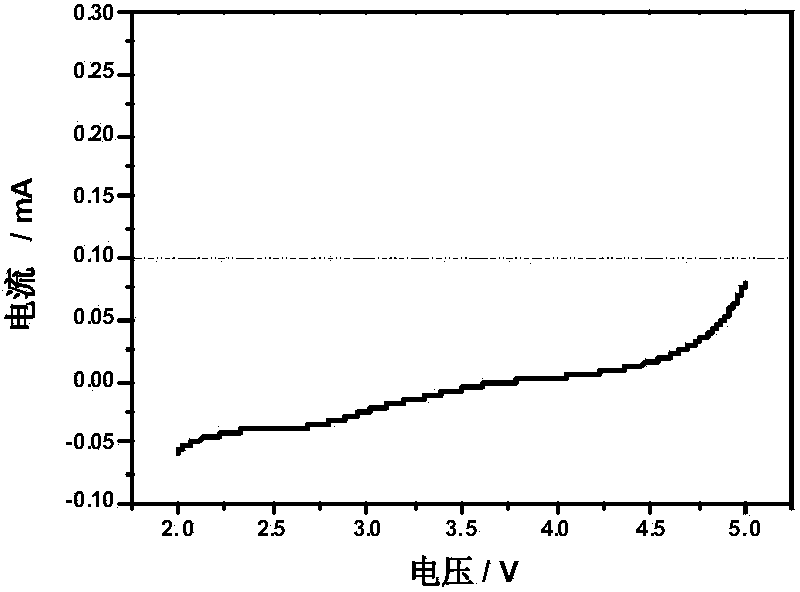
[0029] The ion conductivity measuring method of the film-supported gel polymer electrolyte of the polyvinyl acetal base synthesized by the preparation method provided by the invention is described as follows:
[0030] The gel polymer electrolyte to be tested is clamped with stainless steel sheets to form a battery with ┃stainless steel ┃ polymer film ┃ stainless steel ┃ structure. The AC impedance test is carried out by the electrochemical workstation, and the ionic conductance of the gel polymer film can be calculated according to the following formula Rate σ:
[0031] σ=L / AR.
[0032] Among them, L is the thickness of the membrane, R is the volume resistance of the gel polymer electrolyte obtained by the AC impedance test, and A is the contact area of the gel polymer electrolyte and the stainless steel electrode.
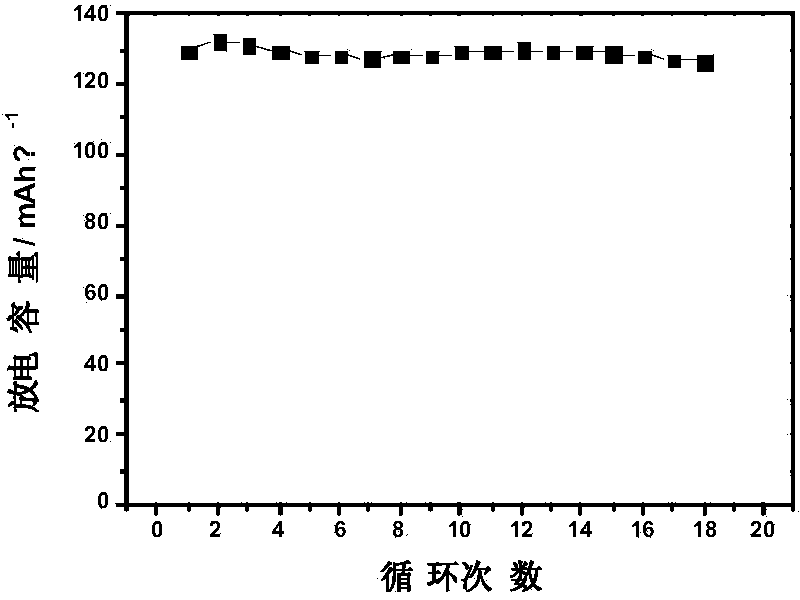
Embodiment 1
[0035] Weigh 0.3g polyvinyl formal (R is a hydrogen atom, molecular weight is 60000-80000, alcoholysis degree is 79%) in a glove box filled with argon gas and add it to 3g N,N-dimethylformamide, Magnetic stirring was used to dissolve completely, and a homogeneous pale yellow clear and transparent solution was prepared. The organic solvent used may be, but not limited to, N,N-dimethylformamide. Add 0.3 g of reactive diluent methyl acrylate monomer to the above solution, and magnetically stir for 5 hours to make it fully mixed. The reactive diluent used can be, but is not limited to, methyl acrylate monomer. Then add 0.01 g of photoinitiator 2-hydroxy-2-methyl-1-phenyl-1-propanone, and stir well to make it evenly mixed. The photoinitiator can be, but is not limited to, 2-hydroxy-2-methyl-1-phenyl-1-propanone. Add the above solution to its total mass, but not limited to, 1.5 times the liquid electrolyte (1mol / L lithium hexafluorophosphate dissolved in a mixed solvent of ethyle...
Embodiment 2
[0037] In a glove box filled with high-purity argon, weigh 0.3g of polyvinyl butyral (R is a propylene group, the molecular weight is about 70,000, and the degree of alcoholysis is 79%) and add it to 4.5g of dimethyl sulfoxide, and stir it magnetically. It was completely dissolved to prepare a homogeneous colorless transparent clear solution. The organic solvent used may be but not limited to dimethyl sulfoxide. Add reactive diluent methyl acrylate monomer 0.2 g to the above solution, and magnetically stir for 5 hours to make it fully mixed. The reactive diluent can be, but is not limited to, methyl acrylate monomer. Subsequently, 0.04 g of photoinitiator benzophenone (BP) was added, and stirred thoroughly for 3 hours. The photoinitiator can be, but is not limited to, benzophenone. Add the above solution to its total mass, but not limited to 2 times the liquid electrolyte (1mol / L lithium hexafluorophosphate dissolved in a mixed solvent of ethylene carbonate and dimethyl car...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com