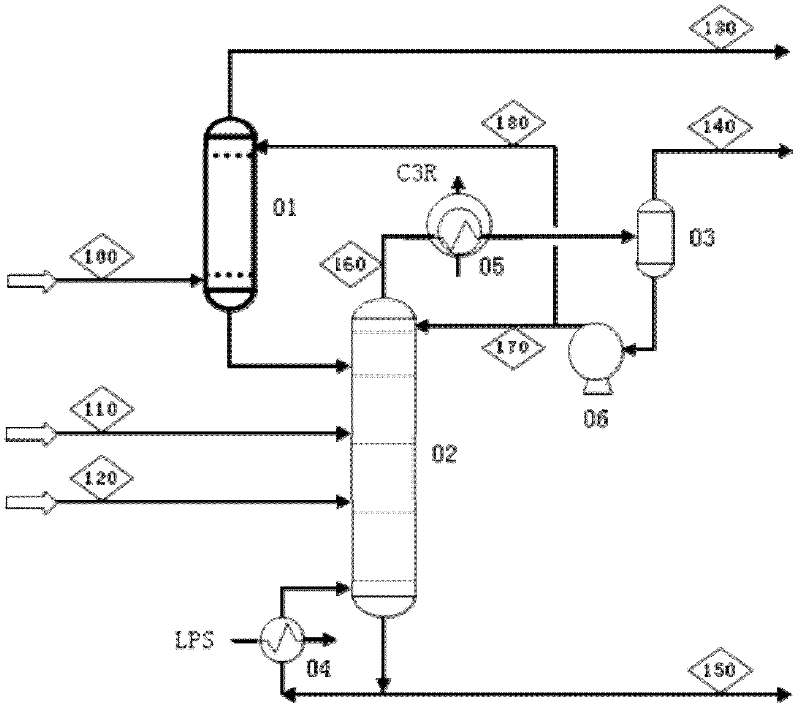
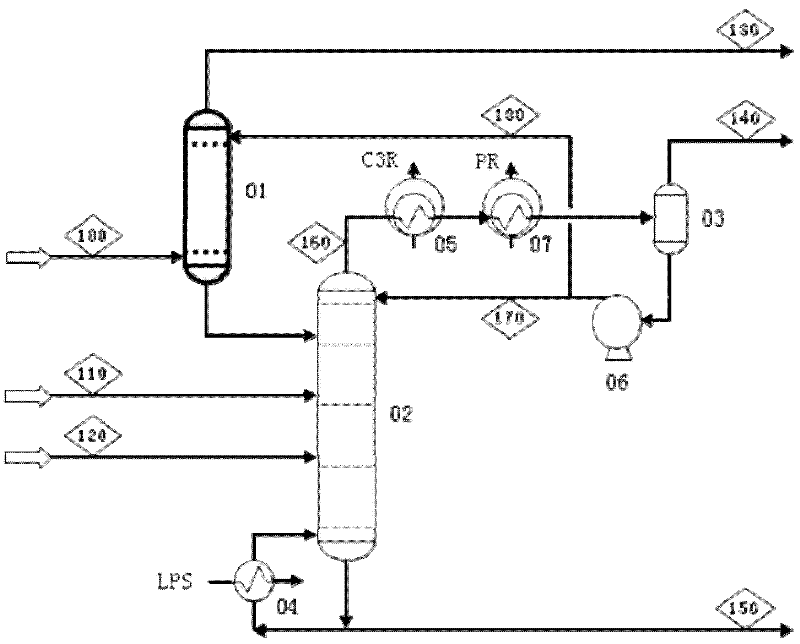
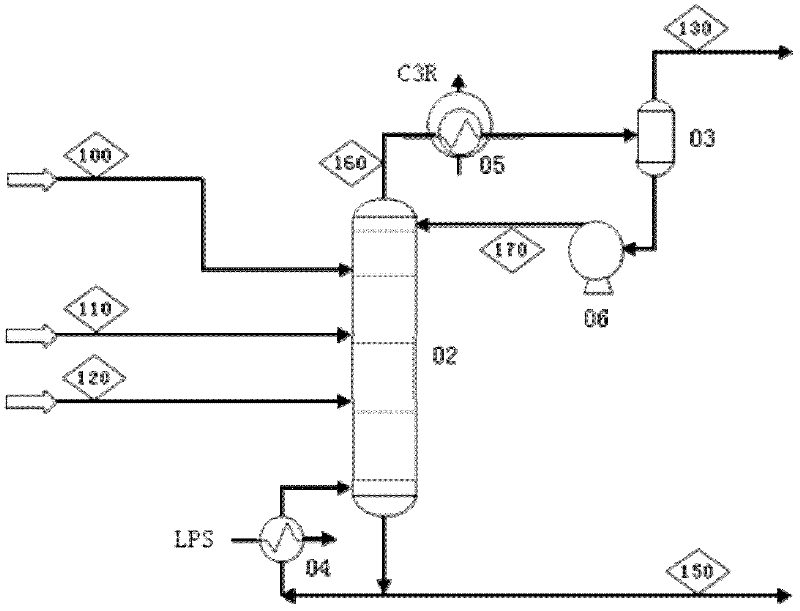
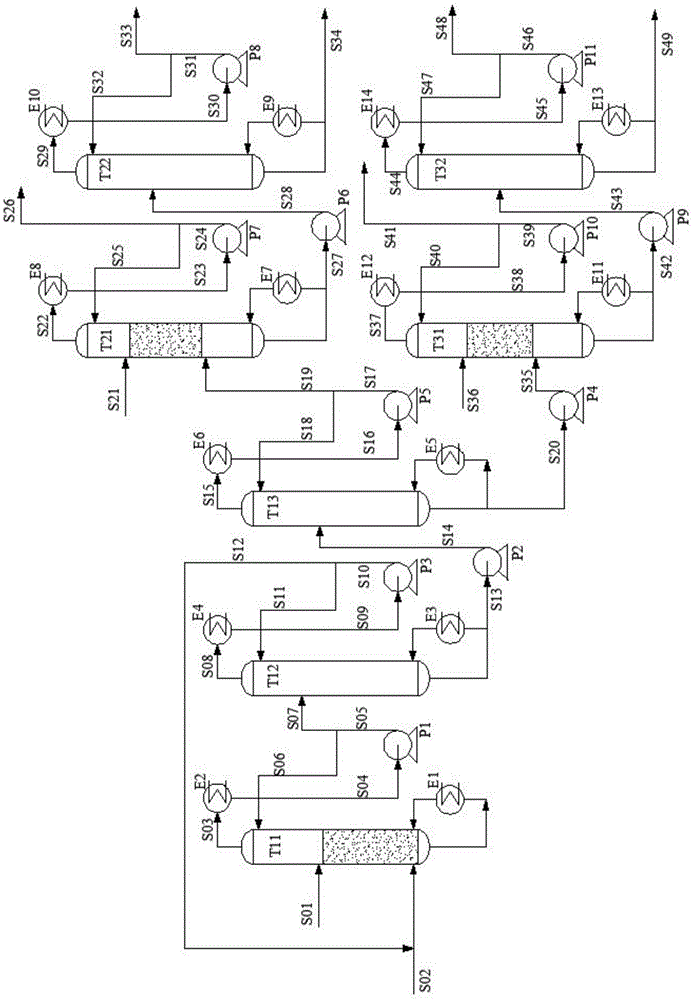
Patents
Literature
146 results about "Relative volatility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Relative volatility is a measure comparing the vapor pressures of the components in a liquid mixture of chemicals. This quantity is widely used in designing large industrial distillation processes. In effect, it indicates the ease or difficulty of using distillation to separate the more volatile components from the less volatile components in a mixture. By convention, relative volatility is usually denoted as α.
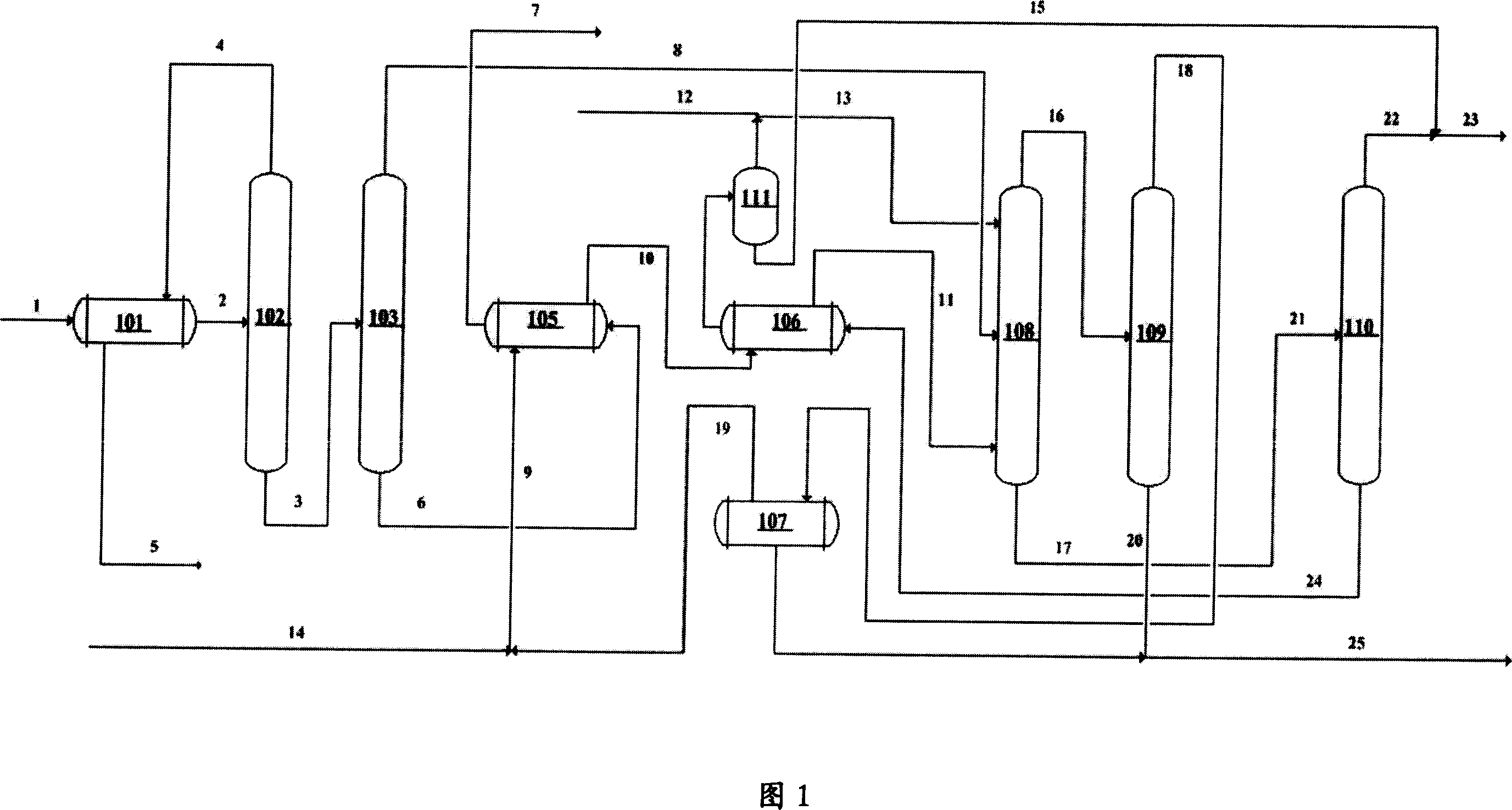
Removal of Water and Methanol from Fluids
ActiveUS20080099400A1Efficient separationPowerfulMembranesUltrafiltrationChemical reactionInternal combustion engine
A method of removing water and / or methanol from fluid mixtures of the water or methanol with other compounds uses vapor permeation or pervaporation of the water or methanol, as the case may be, from the mixture through a membrane having an amorphous perfluoropolymer selectively permeable layer. The novel process can be applied in such exemplary embodiments as (a) removing water or methanol from mixtures of compounds that have relative volatility of about 1-1.1 or that form azeotropic mixtures with water or methanol, (b) the dehydration of hydrocarbon oil such as hydraulic fluid to concentrations of water less than about 50 ppm, (c) removing water and methanol byproducts of reversible chemical reactions thereby shifting equilibrium to favor high conversion of reactants to desirable products, (d) drying ethanol to less than 0.5 wt. % water as can be used in fuel for internal combustion engines, and (e) controlling the water content to optimum concentration in enzyme-catalyzed chemical reactions carried out in organic media.
Owner:COMPACT MEMBRANE SYST INC
Method for separating methyl acetate-methanol mixture by ionic liquid intermittent extractive rectification
InactiveCN102180791AIncrease relative volatilityNo pollution in the processOrganic compound preparationCarboxylic acid esters preparationMethyl acetateMass content
The invention discloses a method for separating a methyl acetate-methanol mixture by ionic liquid intermittent extractive rectification, which is realized in a way that: a rectifier of an extractive rectification tower is adopted, an ionic liquid 1-ethyl-3-methyl imidazolyl acetate is used as an extractant, and the operation is carried out intermittently, so the top of the extractive rectification tower sequentially produces a methyl acetate product of which the mass content is up to 98-99.9%, a methyl acetate-methanol transitional fraction and methanol of which the mass content is 97-99%; and finally, the extractant of which the mass percent is 99% is left in the heating kettle. The invention has the following advantages: the extractant is adopted to enhance the relative volatility of the methyl acetate-methanol system, the purity of the separated methyl acetate product is high, and the ionic liquid used as the extractant is easy to recover and does not pollute the environment; and only one single tower needs to be operated, so that operation is flexible and the equipment investment is low.
Owner:TIANJIN UNIV
Process for producing hexamethylol melamine
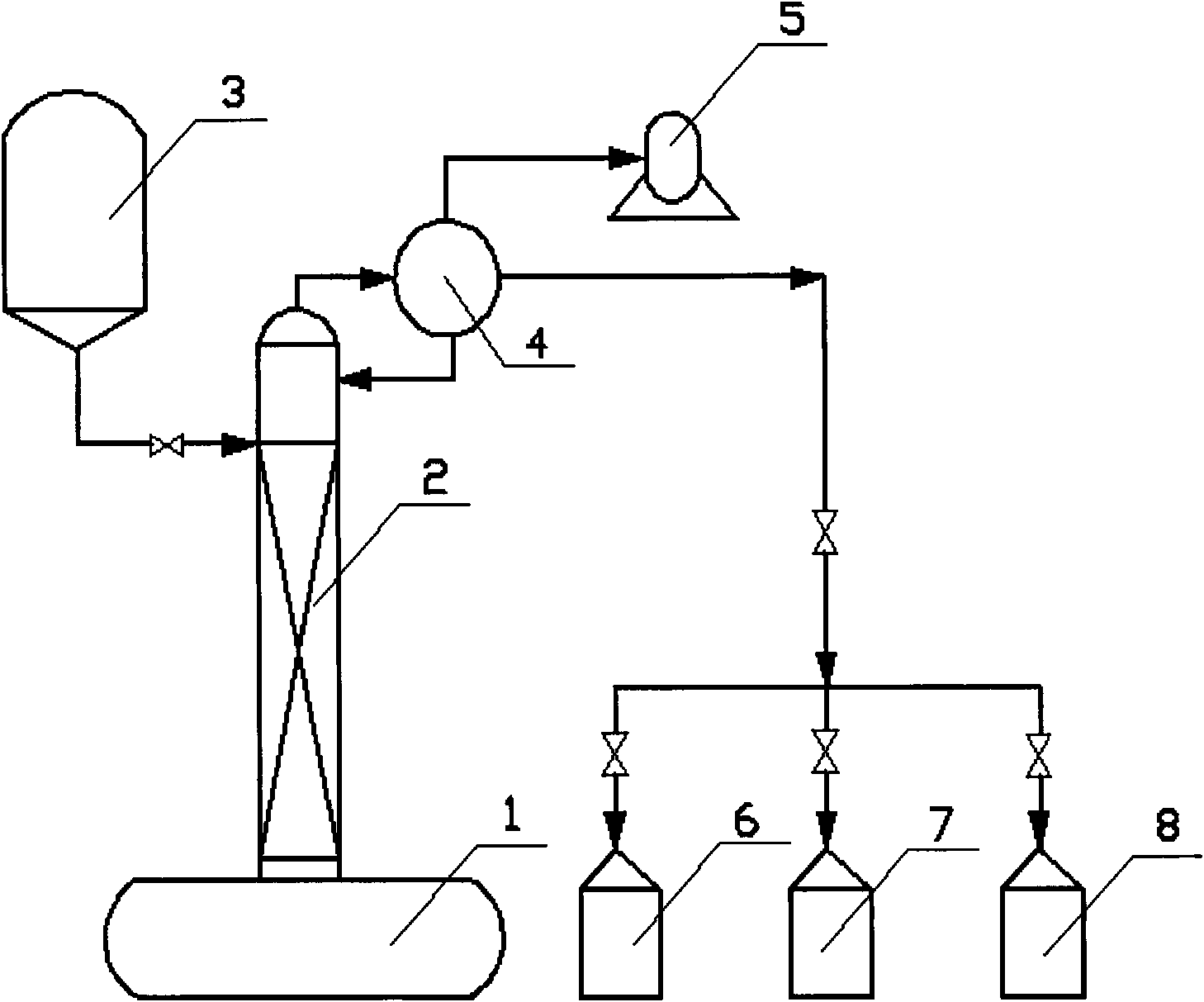
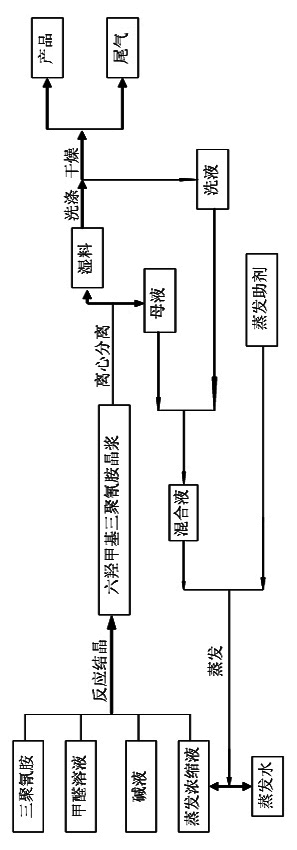
ActiveCN102010380AReduce processingReduce processing difficultyOrganic chemistryWater/sewage treatment by heatingFormateEvaporation
The invention discloses a process for producing hexamethylol melamine, belonging to the field of production and preparation of hexamethylol melamine. The process comprises the steps such as mixing, reaction and crystallization, centrifugalization, evaporation and the like, wherein evaporation auxiliary formate is added when in evaporation so as to change the relative volatility of formaldehyde and water. Furthermore, the process for producing the hexamethylol melamine together with a formaldehyde device can also be used for co-production, so that the concentration of the produced formaldehyde solution is improved to 45wt%-55wt%. The produced hexamethylol melamine provided by the invention has the advantages of small amount of waste water and low formaldehyde content, namely, if 1 ton of hexamethylol melamine is produced, 1 ton of waste water containing 0.84wt% of formaldehyde is discharged. After the process provided by the invention and the formaldehyde device are used for co-production, the formaldehyde production cost and transportation cost are reduced, and the comprehensive cost is 10% less than that of other processes.
Owner:SICHUAN GOLDEN ELEPHANT SINCERITY CHEM CO LTD
Pressure-reducing flow-reversing dual-purpose distillation method and apparatus for refining crude methanol
InactiveCN101130484AReduce energy consumptionIncrease relative volatilityOrganic compound preparationChemical industryReboilerDual purpose
The invention discloses a dual-purpose distilling method and device to refine rough carbinol through decompression adverse current, which consists of a decompression tower (1) and a micro-pressurization tower (7), wherein the decompression tower (1) is front-effect and the micro-pressurization tower (7) is back-effect; the carbinol steaming at top of the micro-pressurization tower is aerated into condensation / reboiler (5) to chill, which can be heat source of tower kettle liquid of the decompression tower. The invention utilizes not only the energy-saving theory of dual-purpose distillation but the character of facilitating to increase relative volatility of relevant architecture of the rough carbinol by pressure reduction efficiently, which reduces energy consumption during refining process to a much more degree.
Owner:CHINA HUANQIU CONTRACTING & ENG CO LTD
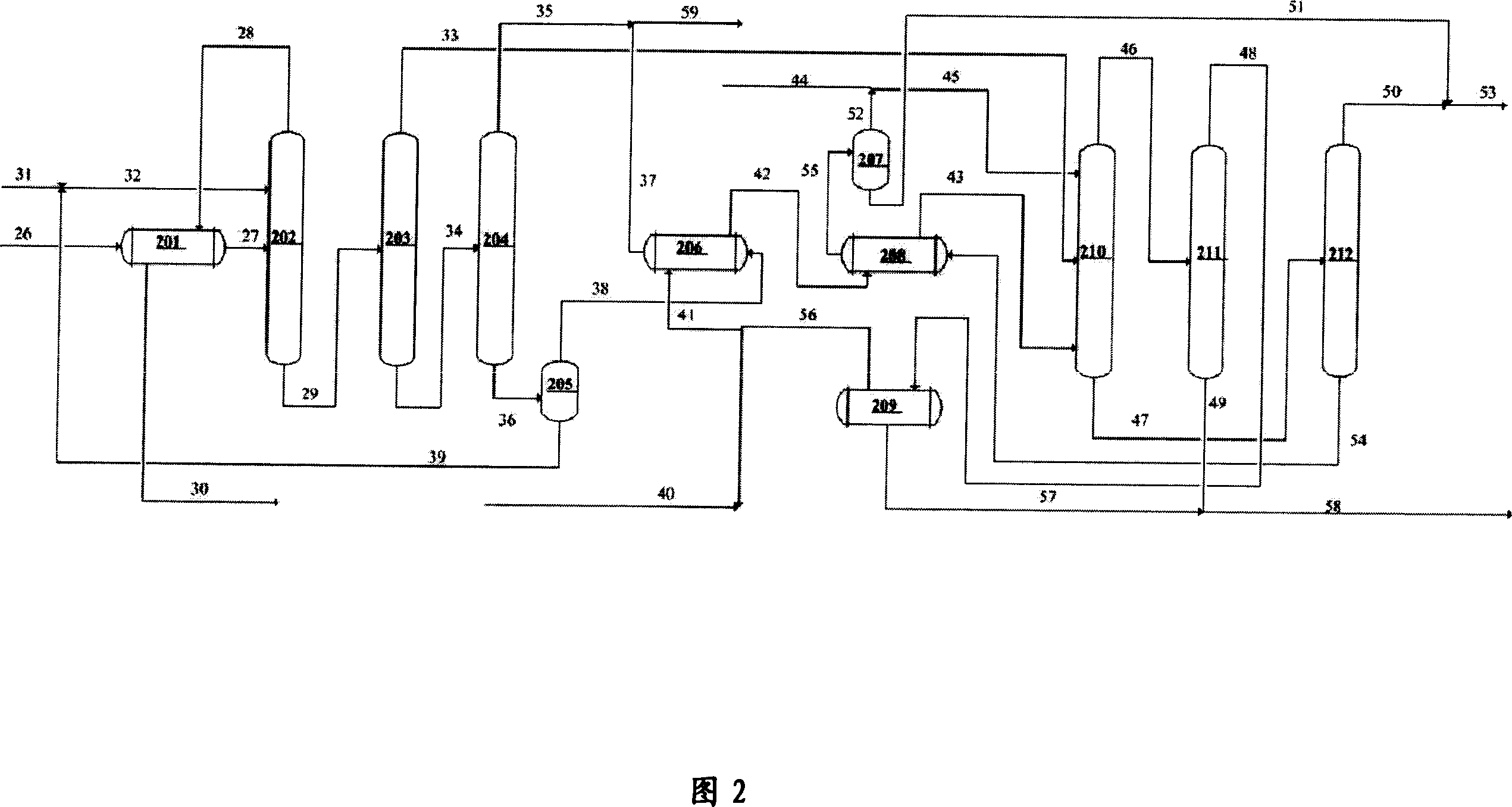
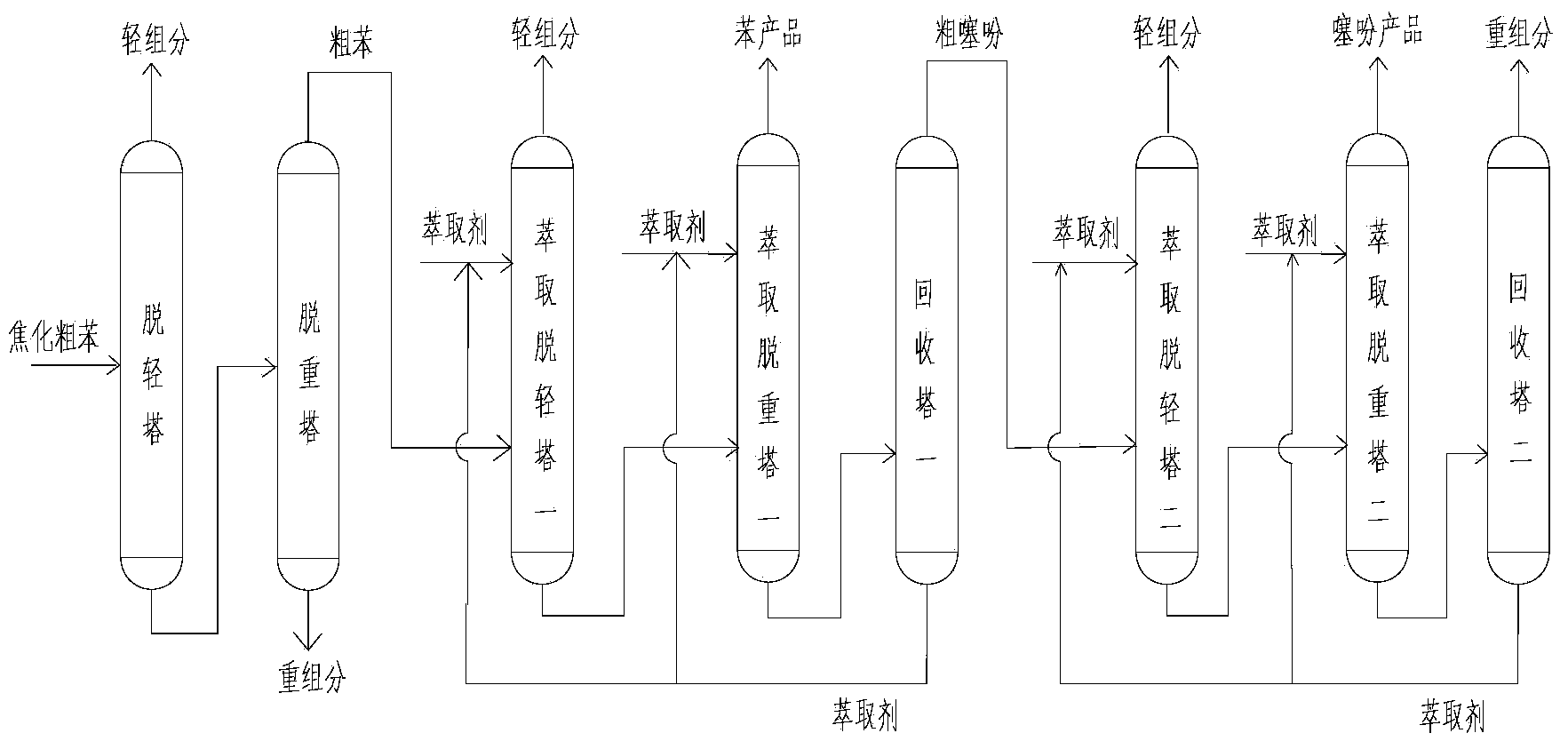
Method of double solvents, benzene substitutive rectification for separating c9 aromatics
This invention discloses a method for separating C9 aromatic hydrocarbons by double-solvent extraction and rectification. The method comprises: (1) pre-fractionating C9 aromatic hydrocarbon mixture, and collecting the rich fraction containing mesitylene and o-methyl ethyl benzene; (2) introducing the rich fraction into the middle of a double-solvent extraction-rectification column, introducing high-boiling-point solvent from the top of the column and low-boiling-point solvent from the bottom of the column, and extracting to obtain mixed fractions containing mesitylene and the low-boiling-point solvent at the top of the column and mixed fractions containing o-methyl ethyl benzene and the high-boiling-point solvent at the bottom of the column; (3) separating the mixed fractions in a solvent recovery column to obtain mesitylene and o-methyl ethyl benzene products, discharging, and recycling the solvents. The method significantly expands the relative volatility between trimethyl benzene and o-methyl ethyl benzene by using high-boiling-point and low-boiling-point solvents.
Owner:CHINA PETROLEUM & CHEM CORP +1
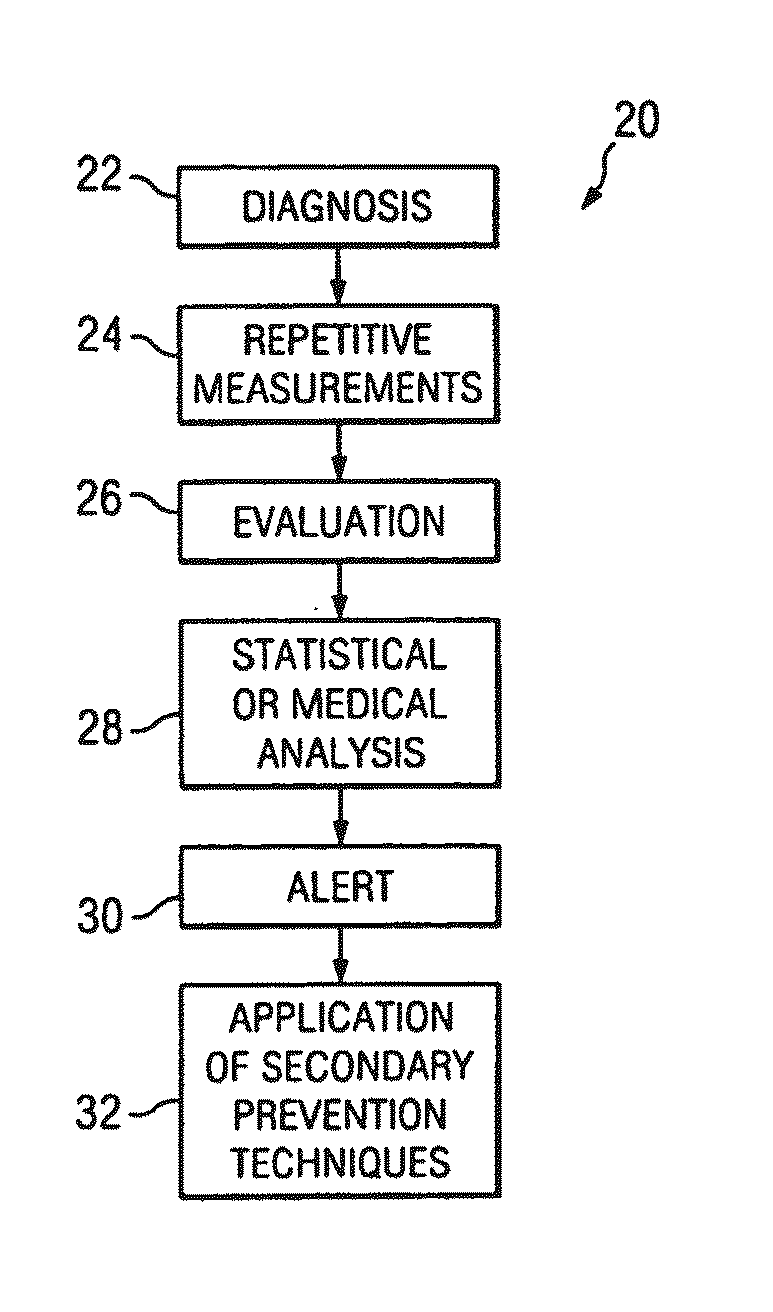
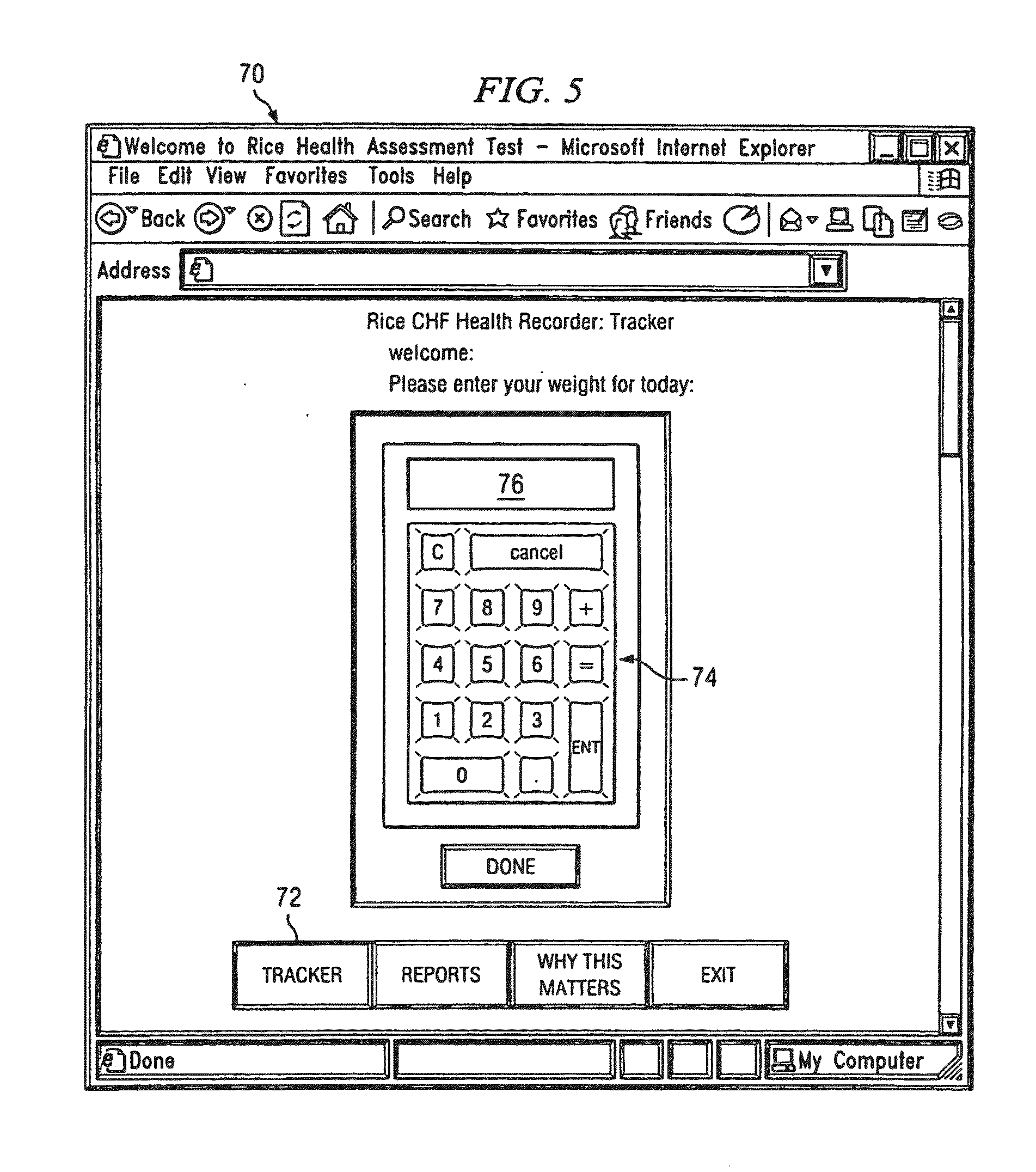
Method and system for identifying volatility in medical data
InactiveUS20110238439A1Easy diagnosisConvenient treatmentMedical data miningData processing applicationsDigital dataData set
A system and method for evaluating the effectiveness of a medical treatment and predicting future medical issues is provided. A digital set of biometric data comprising a plurality of biometric data points is received and stored in a digital database. The digital set of biometric data is analyzed to determine its relative volatility. The relative volatility is then evaluated to help determine the effectiveness of a medical treatment and predict future medical issues.
Owner:RICE WILLIAM H
Method and device for performing refined separation on high-purity yellow phosphorous
ActiveCN101912691AImprove separation efficiencyLow boiling pointVacuum distillation separationPhosphorus compoundsBuffer tankBoiling point
The invention discloses a method and a device for performing refined separation on high-purity yellow phosphorous. A raw material storage tank is connected with a tower bottom; a rectifying tower is positioned above the tower bottom; one end of a condenser is connected with a tower top of the rectifying tower, while the other end is connected with a return tank; the return tank is connected with the tower top of the rectifying tower through a return flowmeter and connected with a front fraction tank and a product tank through a product flowmeter, and a discharge pipe of the product tank is connected with a product collecting tank; a vacuum pump is connected with the condenser, the return tank, the front fraction tank and the product tank through a buffer tank; and a nitrogen tank is connected with the raw material storage tank, the condenser, the return tank, the front fraction tank and the product tank. Through operation of performing rectification under reduced pressure and changinga reflux ratio, the method and the device reduce the boiling point of each component of the material greatly, change the relative volatility among the components, and have the advantages of uniform heating in the tower bottom, simple operation process, high separation efficiency and high purity of the product at the same time.
Owner:湖北兴福电子材料股份有限公司
Methods for improving flow through fluidic channels
Methods for improving fluid flow in one or more flow features of a micro-fluid ejection head and micro-fluid ejection heads having improved fluid flow. One method includes bonding a substrate having a flow feature layer to an ejection head body using a relatively low stress, substantially flexible adhesive containing a relatively volatile polar organic compound. The adhesive is cured under conditions sufficient to induce outgassing of at least a portion of the relatively volatile polar organic compound on at least a portion of a flow feature surface sufficient to increase fluid wetting of the flow feature surface.
Owner:FUNAI ELECTRIC CO LTD
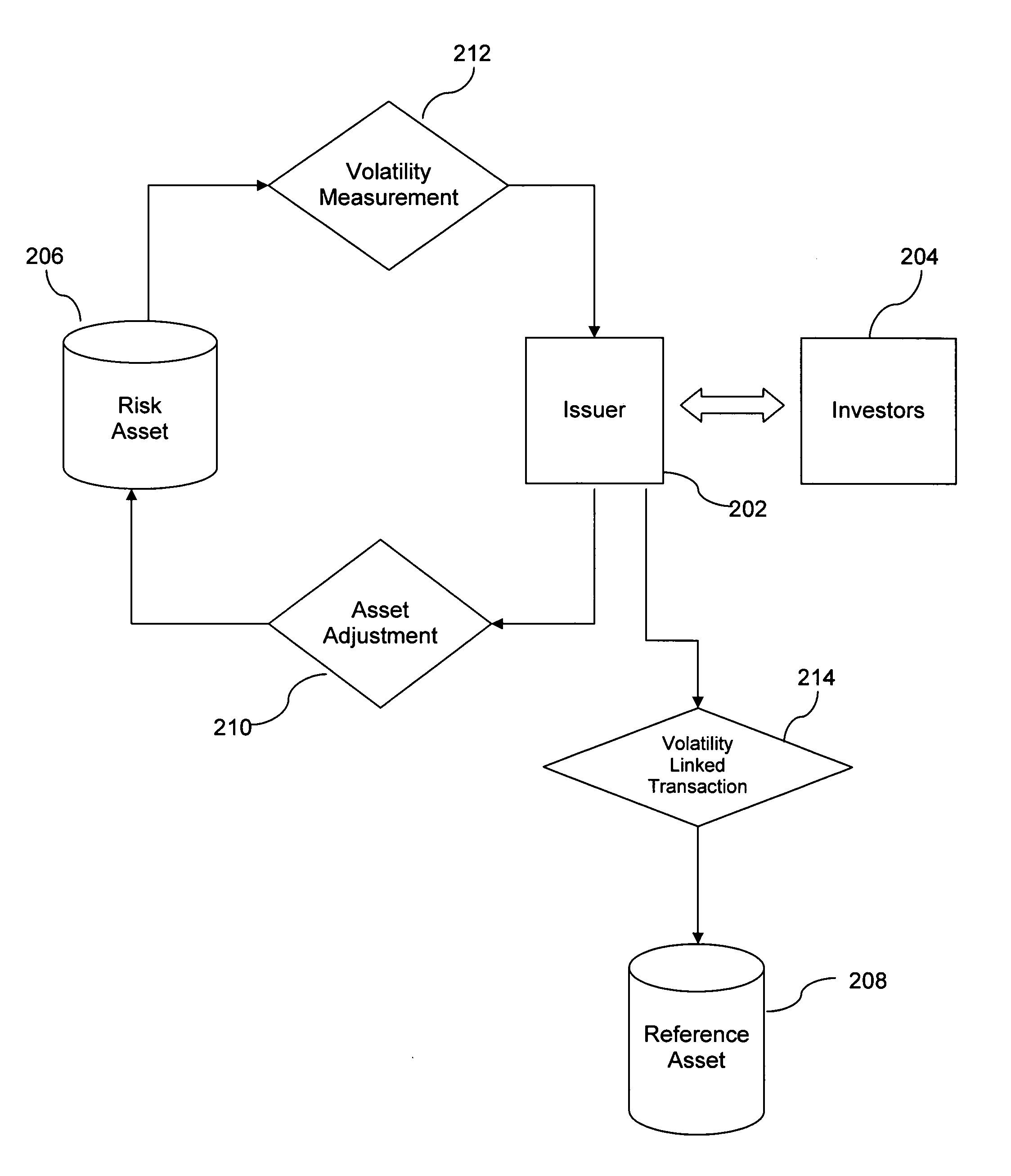
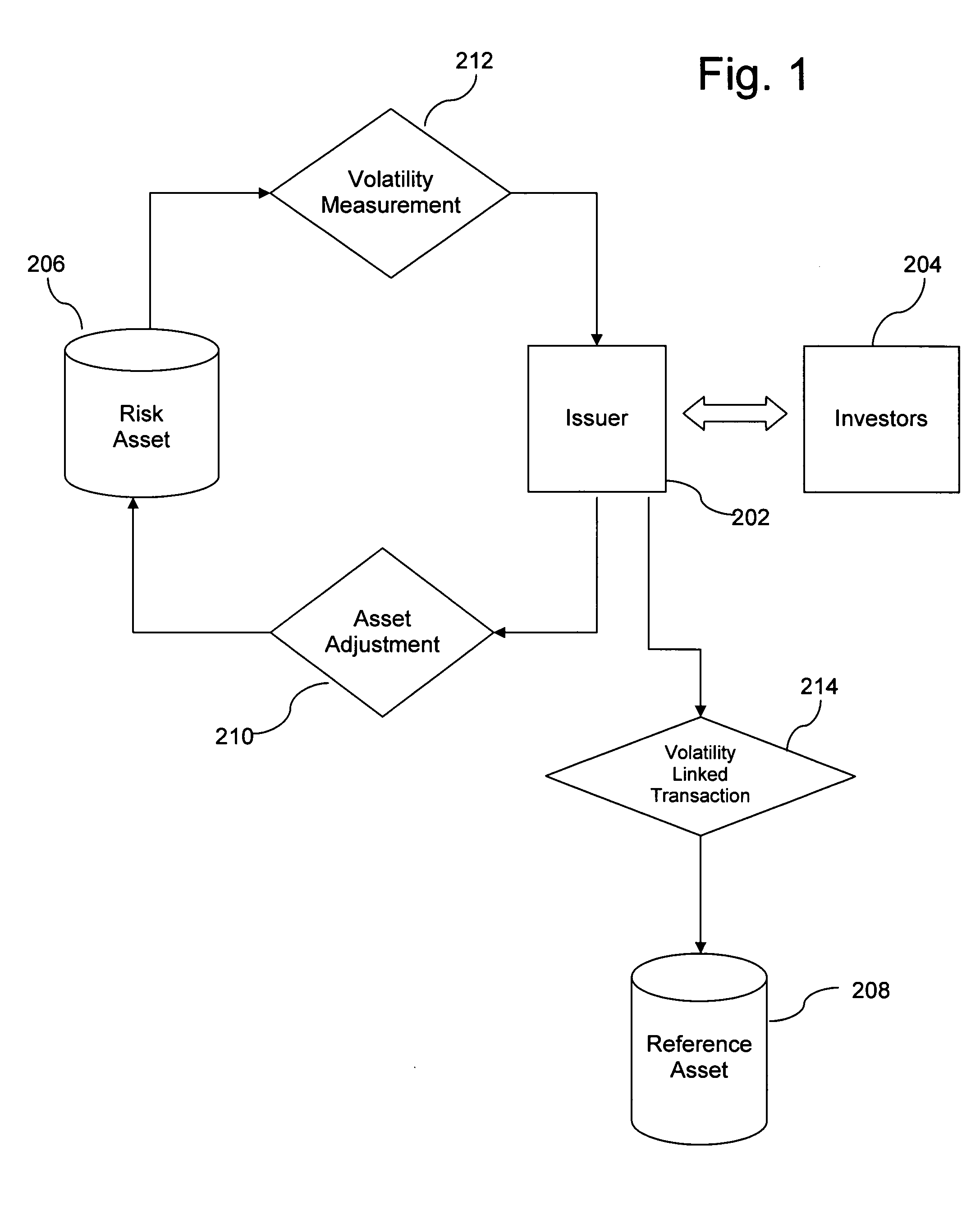
System and method for relative-volatility linked portfolio adjustment
InactiveUS20060271452A1Effectively transfer hedging of its volatility riskIncrease opportunitiesFinanceDerivatives marketEngineering
Owner:SPARAGGIS PANAYOTIS TAKIS
Extraction and separation method for hydrocarbons mixture based on ionic liquor
The present invention is C4 hydrocarbon mixture extracting and separating process. Ionic liquid additive is introduced to form one new type of composite extractant for extracting and separating C4 hydrocarbon mixture with 1, 3-butadiene as the main component. Introducing the ionic liquid additive into the extractant can increase the total dissolubility of C4 hydrocarbon mixture in the extractant, raising the hydrocarbon selectivity and relative volatility, and thus raise the extracting and separating effect obviously. In addition, the added ionic liquid has very low vapor pressure and high boiling point, and thus convenient recovery and reuse.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method for refining petrobenzene and thiophene by using ionic liquid complex solvent
InactiveCN103664480AImprove thermal stabilityLow steam pressureExtraction purification/separationBulk chemical productionSolventRelative volatility
The invention creates and provides a method for refining petrobenzene and thiophene by using an ionic liquid complex solvent. Coking crude benzene is pretreated to form crude benzene in a pretreatment procedure, crude benzene is extracted and rectified twice in the ionic liquid complex solvent during a benzene refining procedure to form a 99.99% benzene product and crude thiophene, a thiophene product with purity of more than 99.7% is also obtained by extracting and rectifying crude thiophene twice with the ionic liquid complex solvent in the thiophene refining procedure, yield is more than 97%, and all the ionic liquid complex solvent is recycled after being refined and recycled through a recovery tower. In the method, the quality of the benzene product reaches A-level standard of petrobenzene national standard, the benzene product can substitute petrobenzene in use, meanwhile, thiophene is recycled efficiently, and the economic benefit increased therefrom is more than 50%; the ionic liquid has good thermal stability, low vapor pressure and high polarity, the complex solvent formed by the ionic liquid has high selectivity, relative volatility among the constituents is increased, dosage of an extraction agent is reduced, and process energy consumption and production cost are respectively reduced by 25% and 40%, so that energy-saving effect is remarkable.
Owner:TIANJIN CLEANTECH TECH
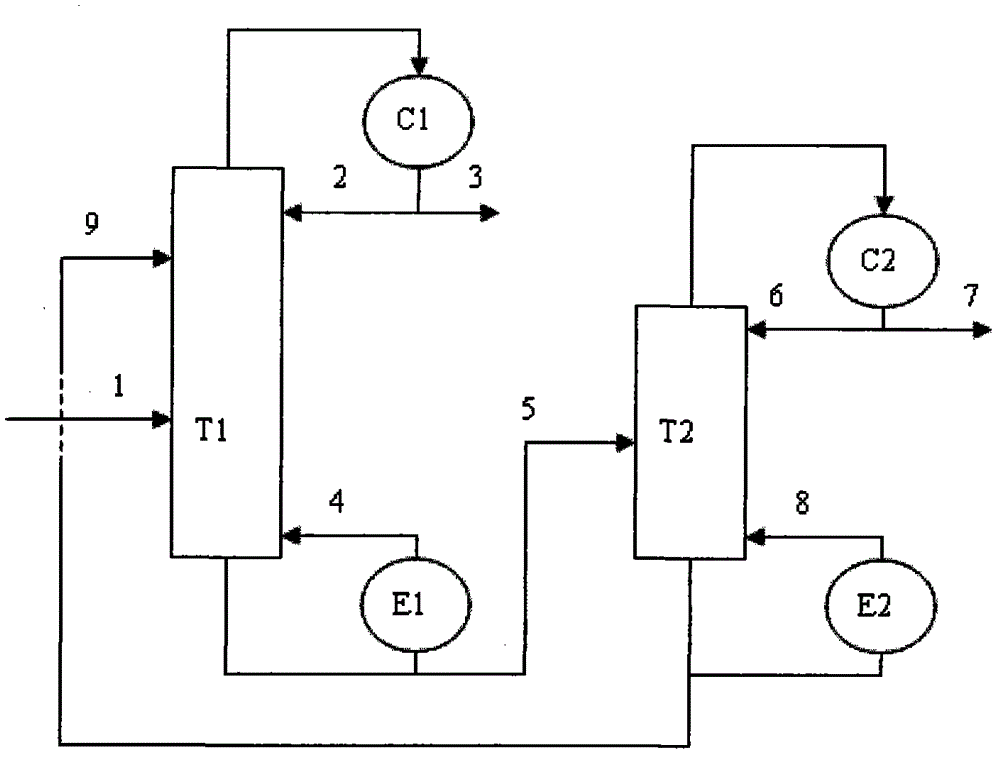
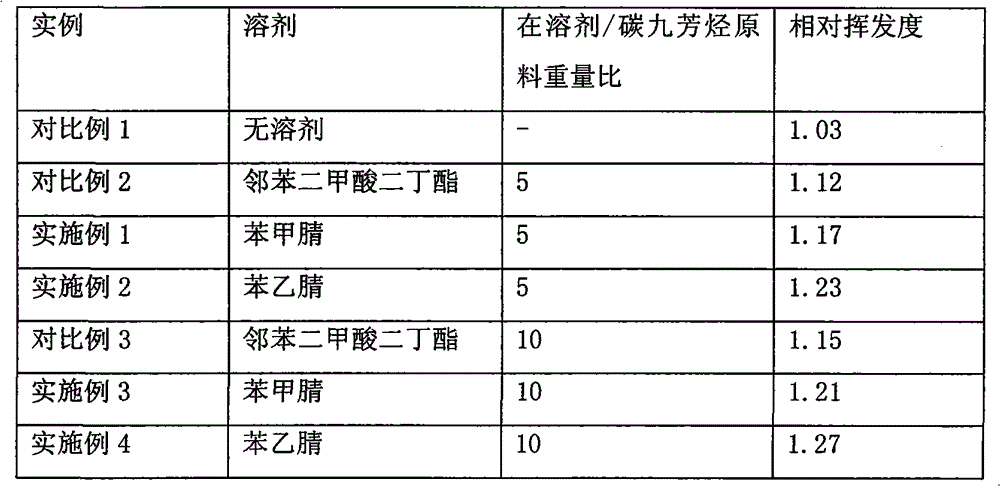
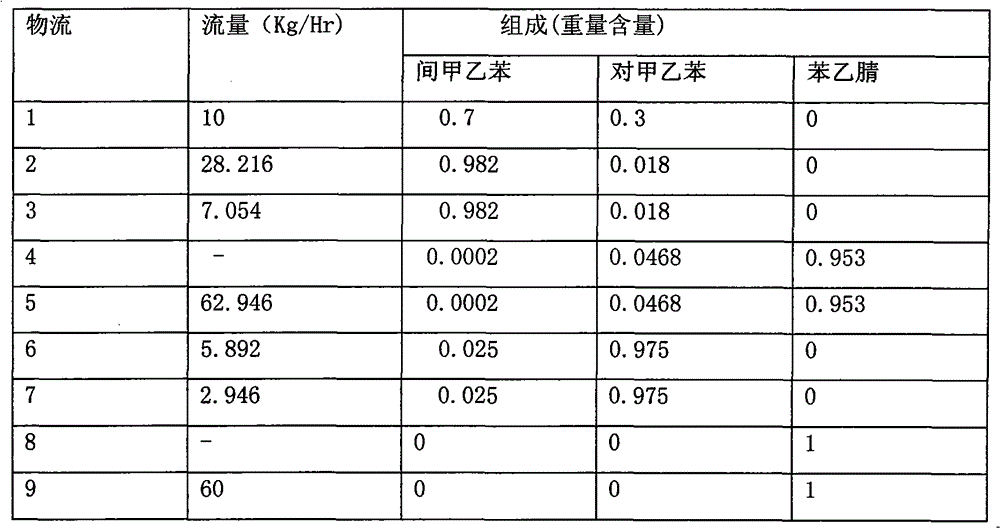
Method for separating m-ethyltoluene and p-ethyltoluene by extractive distillation
ActiveCN102795957AImprove separation efficiencyIncrease relative volatilityDistillation purification/separationBenzeneExtractive distillation
The invention discloses a method for separating m-ethyltoluene and p-ethyltoluene by extractive distillation. The extractive distillation solvent adopted by the method is a nitrogenous compound, preferably cyanobenzene or benzyl cyanide, and more preferably benzyl cyanide. The method comprises the following steps: introducing an m-ethyltoluene / p-ethyltoluene mixture from the middle of the extractive distillation tower, and introducing an extractive distillation solvent from the tower top; and after carrying out extractive distillation, discharging the m-ethyltoluene from the top of the extractive distillation tower, discharging the rich solvent containing rich p-ethyltoluene from the tower bottom, sending the rich solvent into a solvent recovery tower, discharging the p-ethyltoluene from the top of the recovery tower, and recycling the extractive distillation solvent discharged from the bottom of the recovery tower. The invention has the advantages of low operation energy consumption and high product purity; and the solvent adopted by the method can obviously improve the relative volatility between m-ethyltoluene and p-ethyltoluene, is easy to recycle, and has high chemical thermal stability.
Owner:CHINA PETROLEUM & CHEM CORP +1
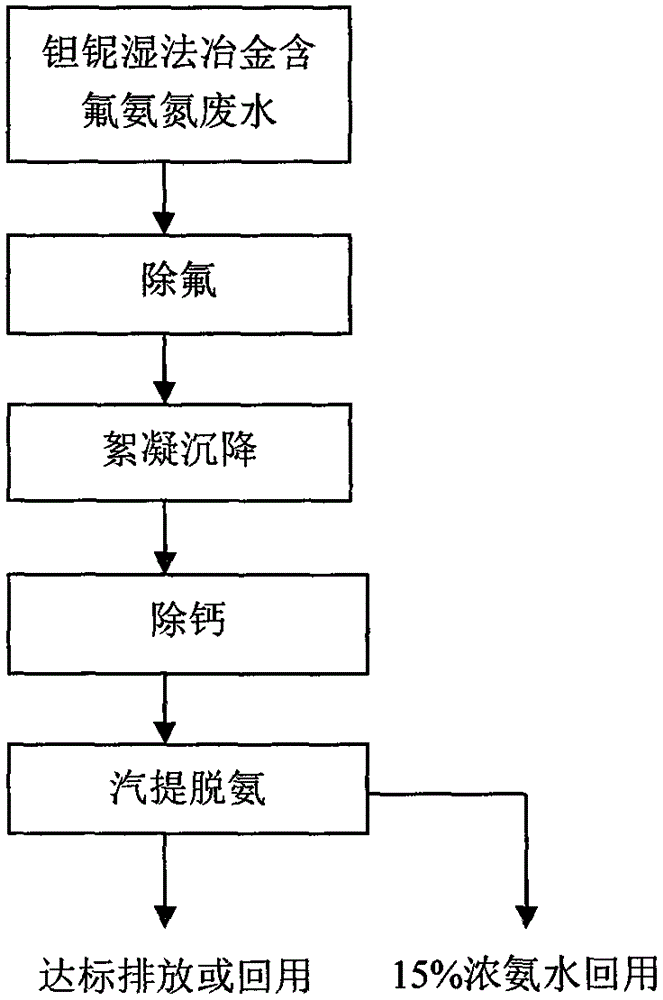
Treatment technology of waste water containing fluoride and ammonia nitrogen
ActiveCN106746102AReduce pollutionImprove utilization efficiencyTreatment involving filtrationWaste water treatment from metallurgical processNiobiumEmission standard
The invention provides a treatment technology of waste water containing fluoride and ammonia nitrogen. The technology is used for treating waste water containing fluoride and ammonia nitrogen in tantalum-niobium hydrometallurgy. The technology is characterized in that the relative volatility difference between ammonia and water is utilized; an ammonia-water separating technology in which high-efficiency distillastion is treated as the main technology core is carried out and combined with a pre-treatment technology to perform defluorination-calcium removing-strong dissolving and complexing-molecular distilling, so as to realize the removal of fluorine and ammonia; the drained water obtained after treatment reaches national primary emission standard; meanwhile, high-purity ammonia water with concentration being equal to or greater than 15% is recovered for production. According to the technology, the ammonia is recycled, so that the balance of payments of the treatment cost of the ammonia containing waste water can be achieved; the requirement on comprehensive utilization of the resource can be met; the technology brings a certain economic benefits.
Owner:稀美资源(广东)有限公司
Method, process and apparatus for separation of ethylene glycol and 1,2-butanediol
ActiveCN105622338AHigh purityEfficient separationOrganic compound preparationHydroxy compound separation/purificationLiquid productBoiling point
The invention relates to a method, a process and an apparatus for separation of ethylene glycol and 1,2-butanediol. The method includes, (1) subjecting ethylene glycol and 1,2-butanediol to reaction through acetal / ketal to produce acetal / ketal liquid product mixture correspondingly, (2) separating the acetal / ketal liquid product mixture containing ethylene glycol and 1,2-butanediol by a series of rectifying columns, (3) separating different acetal / ketal products by rectifying according to difference of boiling points of acetal or ketal, (4) respectively hydrolyzing the acetal / ketal products to obtain an ethylene glycol primary product and a 1,2-butanediol primary product, and (5) purifying the ethylene glycol primary product and the 1,2-butanediol primary product respectively by rectifying to obtain an ethylene glycol product and a 1,2-butanediol product. The purity of the ethylene glycol product can be up to 99.9% and the recovery thereof can be up to 99.5%; the purity of the 1,2-butanediol product can be up to 98.5%. By a reversible-reaction conversion method, the difficulty in separating ethylene glycol and 1,2-butanediol which are of similar boiling points and low relative volatility and are azeotropic is changed into the problem about separation of acetal / ketal products which are easy to separate relatively.
Owner:TIANJIN UNIV
C<2->/C<3+> light dydrocarbon separation method integrating absorption method with fractionation method and C<2->/C<3+> light dydrocarbon separation system
InactiveCN102643153AIncrease relative volatilityReduce separation energy consumptionDistillation purification/separationAbsorption purification/separationHydrogenFractionation
The invention relates to a C<2-> / C<3+> light dydrocarbon separation method integrating an absorption method with a fractionation method and a C<2-> / C<3+> light dydrocarbon separation system, which are used for separating C<2-> from C<3+> in the process of front-end deethanization. The method comprises the following steps of: separating most C<2-> including light components such as hydrogen and methane in charging material by an absorption tower, and fractionating the residual C<2-> and all C<3+> by a fractionating tower. Absorbent of the absorption tower is 'barren liquor' from the inner part of a system, i.e. some part of liquid phase in a reflow tank of the fractionating tower. Compared with the single fractionation method, the method integrating the absorption method with the fractionation method can save more energy, and can enhance the relative volatility bwtween the light key component and the heavy key component since a deethanization tower adopts lower tower pressure, so that the aim of reducing separation energy consumption can be achieved. When the fractionating tower adopts lower tower pressure from 22-26 Bar (a), the separation energy consumption of the whole system can be obviously lower than that of the prior art only by one more heat exchanger using refrigerant from -47 DEG C to -52 DEG C.
Owner:CHINA HUANQIU CONTRACTING & ENG CO LTD
Reaction-rectification-separation-refinement novel method, technique and device of ethylene glycol and 1,2-butanediol
ActiveCN105541551AHigh purityEfficient separationOrganic compound preparationChemical industryKetoneReversible reaction
The invention relates to a separation method, technique and device of ethylene glycol and 1,2-butanediol. The method comprises the following steps: reacting a diol mixture and aldehyde or ketone in a reaction rectification tower by acetal or ketal reversible reaction to generate an acetal / ketone mixture, rectifying to separate the acetal / ketone mixture, and carrying out reaction, rectification and hydrolysis to obtain the high-purity ethylene glycol and 1,2-butanediol products. By using the technique for separation and purification, the purity of the main product ethylene glycol can reach 99.9% or above, the recovery rate can reach 99.5% or above, and the purity of the 1,2-butanediol product can reach 98.5% or above. By using the reaction rectification method, the difficulty in separating azeotropic ethylene glycol and 1,2-butanediol with approximate variable boiling point and low relative volatility becomes the problem of separation of the separable acetal / ketone product, thereby effectively implementing the high-efficiency separation on the ethylene glycol and 1,2-butanediol.
Owner:TIANJIN UNIV
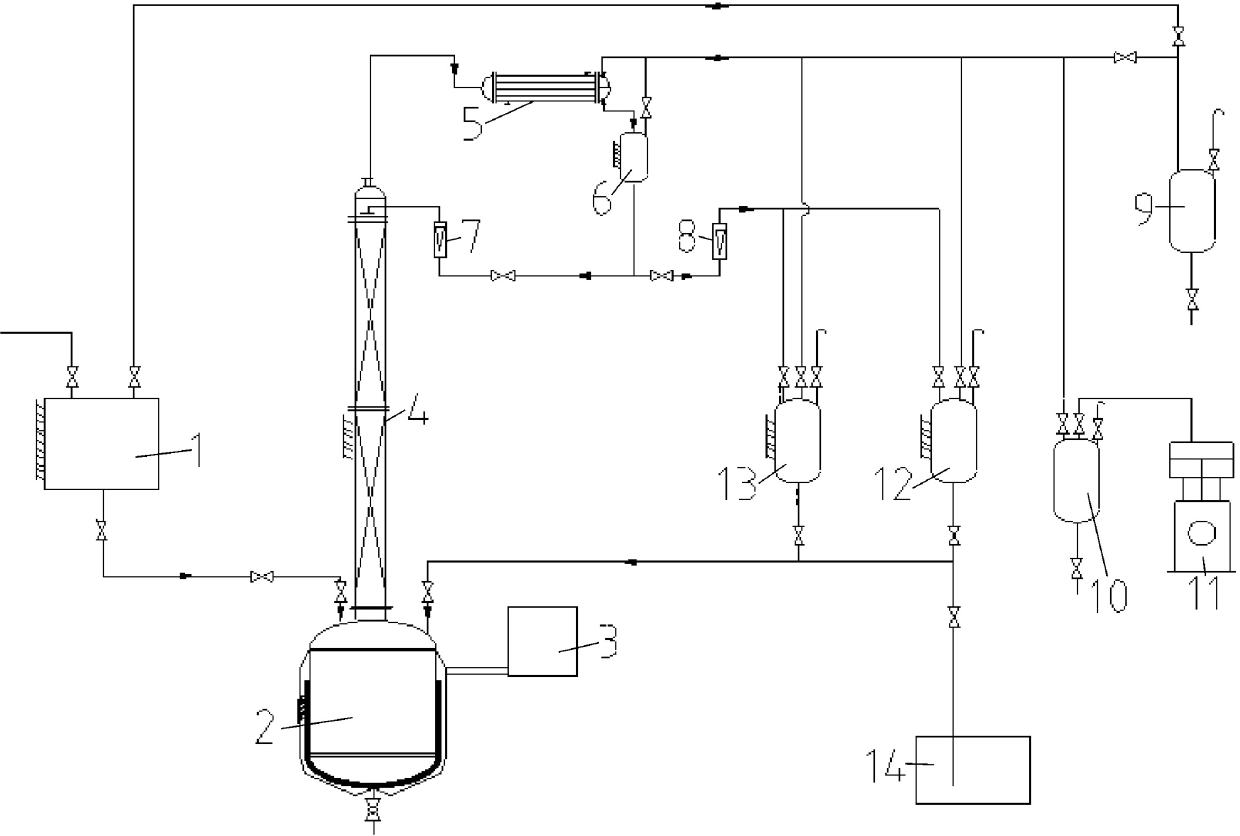
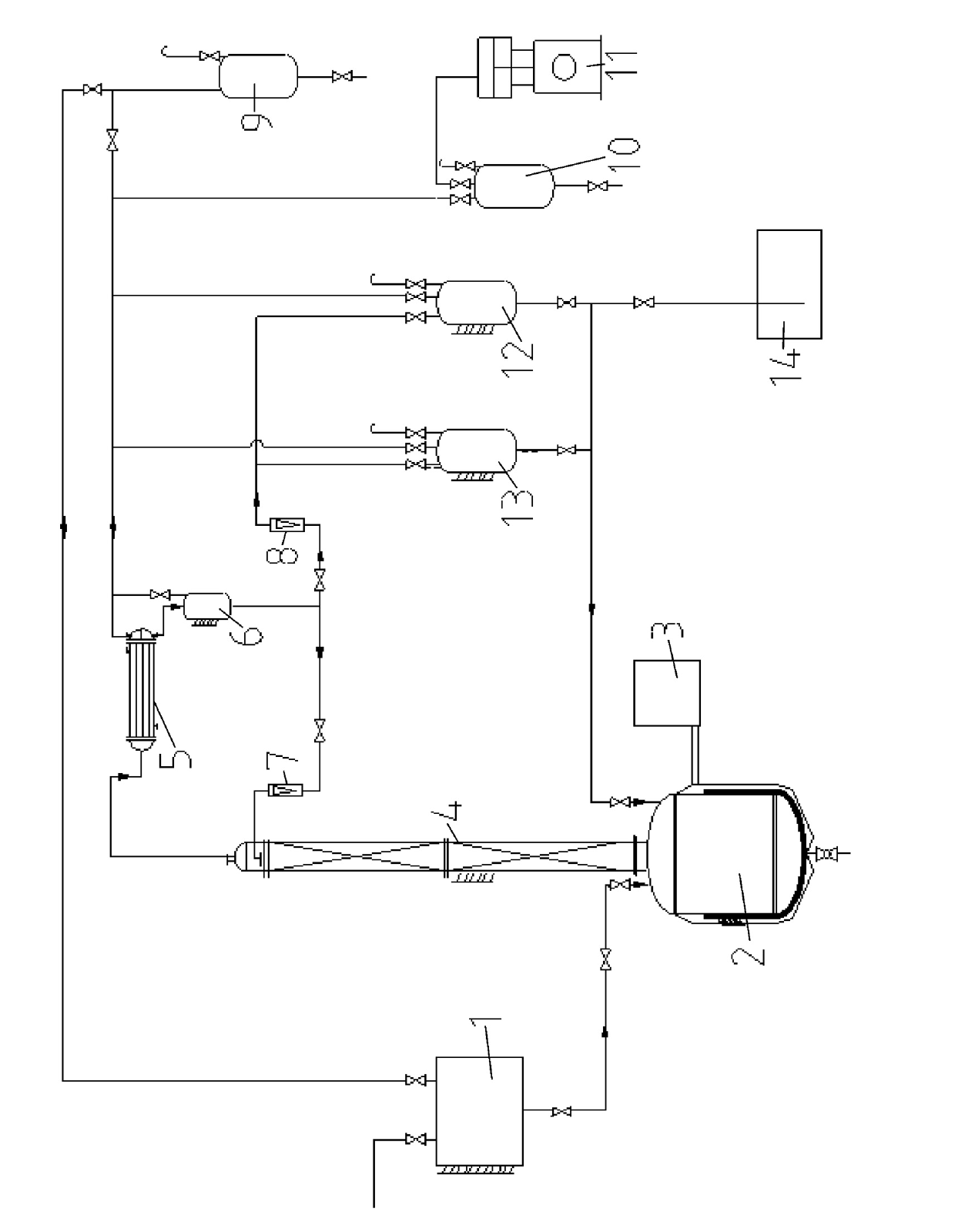
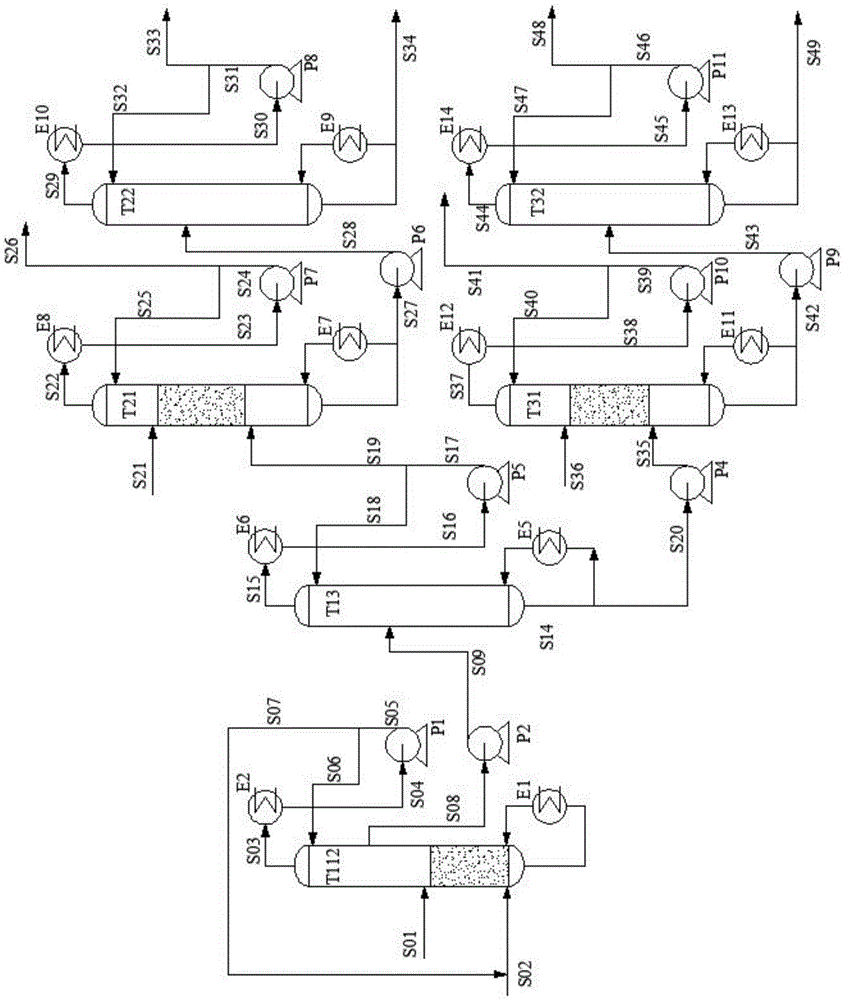
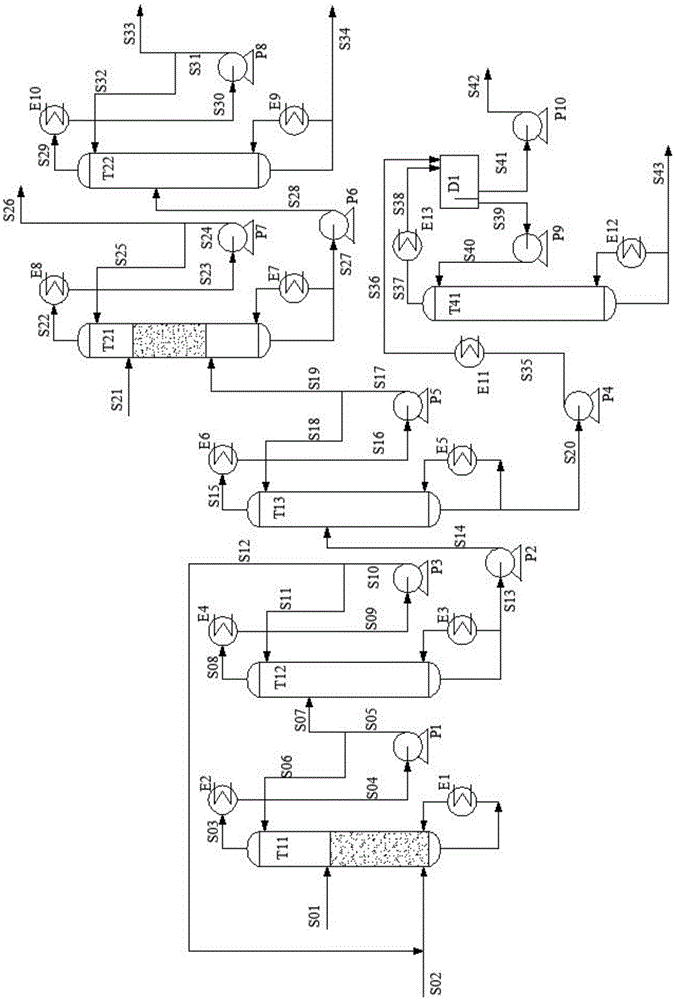
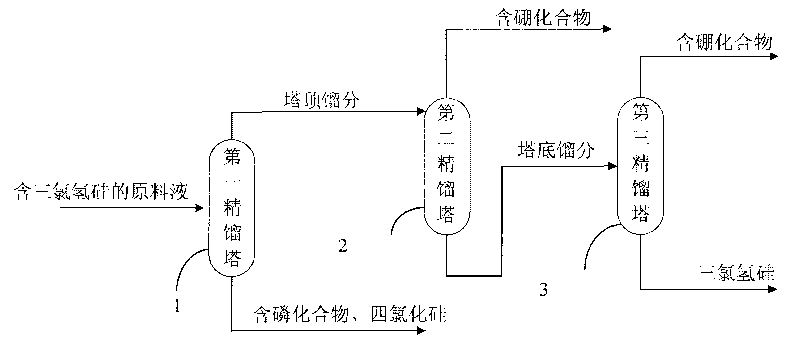
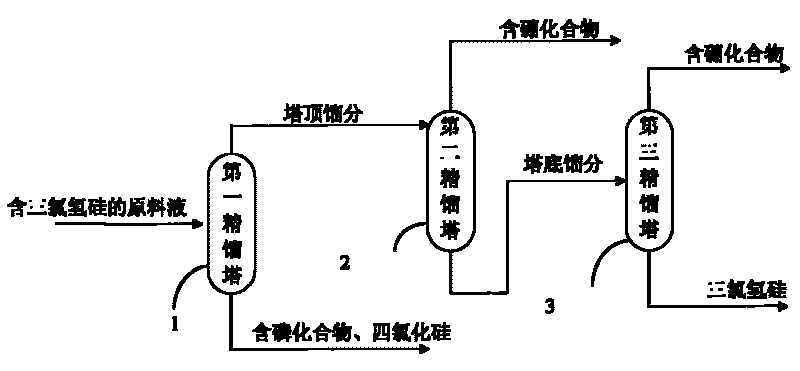
Method and device for purifying trichlorosilane
The invention discloses a method for purifying trichlorosilane, which comprises the following steps: a) sending raw material liquid containing trichlorosilane into a first distillation column for distillation and separating a first part of impurities from the raw material liquid, wherein the relative volatility of trichlorosilane relative to the first part of impurities is 1.6 to 2.5; b) sending overhead fractions of the first distillation column into a second distillation column for distillation and separating a second part of impurities from the overhead fractions, wherein the relative volatility of the second part of impurities relative to trichlorosilane is 1.4 to 1.7; and c) sending column-bottom fractions of the second distillation column into a third distillation column for distillation and separating the second part of impurities from the column-bottom fractions, wherein the reflux ratio of the third distillation column is greater than that of the second distillation column. Experimental results show that the boron content of trichlorosilane can be reduced to 0.01 ppba and the phosphorus content of trichlorosilane can be reduced to 0.1 ppba according to the method provided by the invention.
Owner:GUODIAN NINGXIA SOLAR
Normal hexane and benzene extractive distillation operating method
The invention relates to a normal hexane and benzene extractive distillation operating method. The device comprises a normal hexane separation extraction and rectification and purification system and a benzene extractive distillation rectification and purification system. The compounded N-methylpyrrolidone solvent or N-formyl morpholine solvent serves as an extraction agent, so that the relative volatility of aromatic hydrocarbon and non-aromatic hydrocarbon is improved, and the relative volatility of methyl cyclopentane and normal hexane is improved; two extracting agent recovery towers are in negative pressure operation, and the tower kettle temperature is relatively low; liquid-liquid phase does not occur in the two extracting agent recovery towers in the operation process, and the operation is stable; the compounded N-methylpyrrolidone or N-formyl morpholine extracting is recovered and cooled and enters the extraction and rectification tower; and the benzene, toluene and non-aromatic mixtures are heated and enter the benzene extraction and rectification tower. The benzene content in the normal hexane product obtained by the operation method is lower than 100ppm, the purity of the normal hexane product is over 99.0 weight percent, and the purity of benzene product is over 99.9 weight percent.
Owner:TIANJIN UNIV
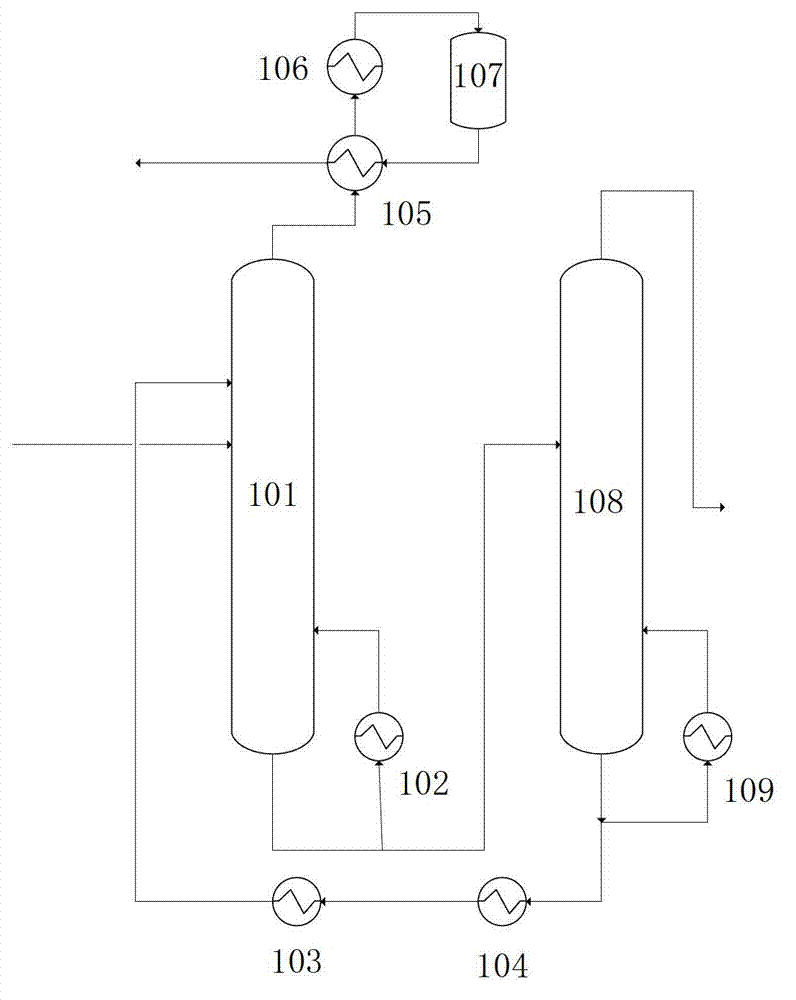
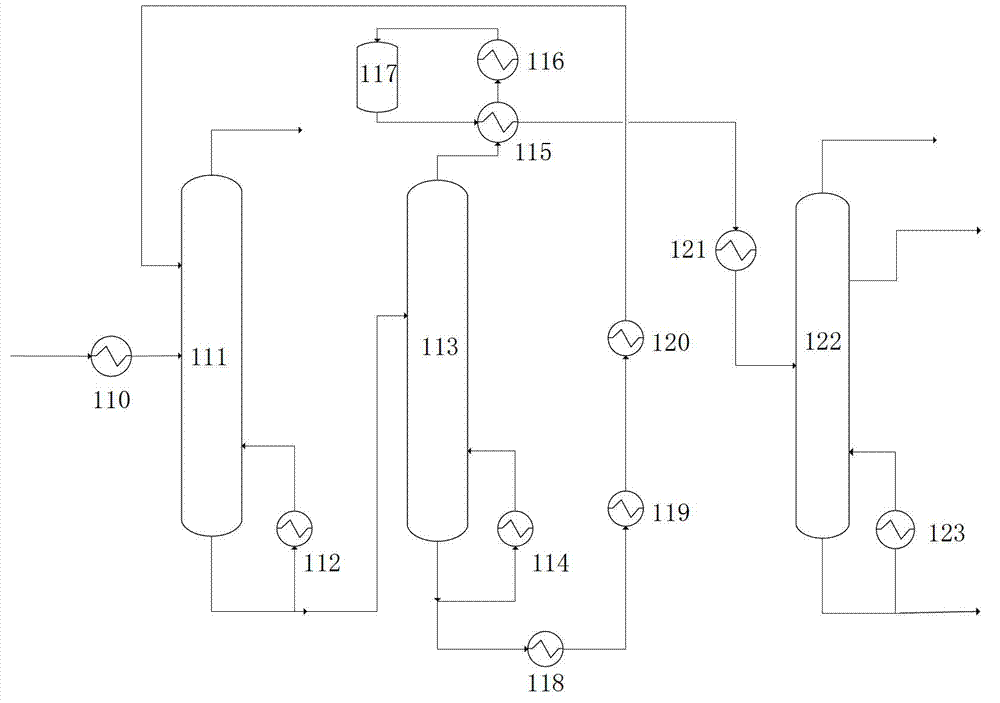
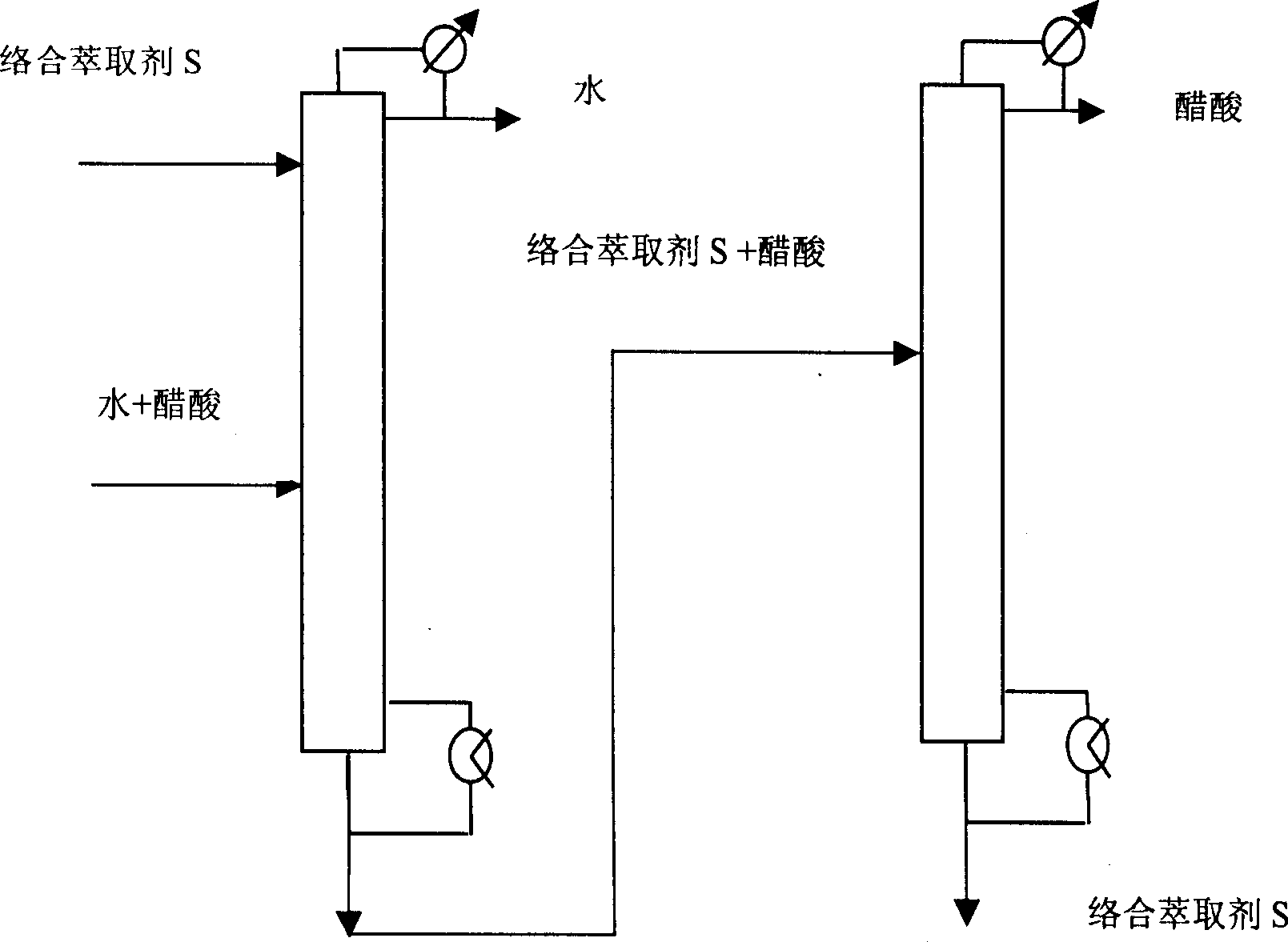
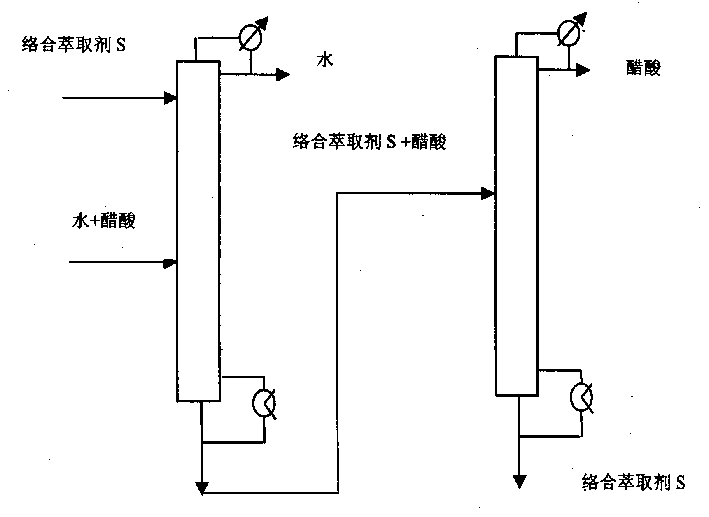
Method for separating acetic acid and water by complexation extraction and rectification
InactiveCN1405137AIncrease relative volatilityImprove energy savingCarboxylic compound separation/purificationTheoretical plateAcetic acid
THe invention provides a method for separating acetic acid and water by complexation, extraction and rectification process. Said invention utilizes the acting force between organic alkali and acetic acid, which is stronger than that of water, and greatly raises the relative volatilities of acetic acid and water so as to separate them. In the fractionating recrtifying tower with 10-50 theoretical plates it adopts tertiary amine organic alkali as complexation extracting agent, under the condition of normal pressure (or reduced pressure), its solvent ratio is 0.5-5 and refluxing ratio is 0-5, the water whose mass fraction is greater than 99.7% can be obtained from tower top, and in the solvent recovering tower with 5-40 the oretical plates, under the condition of reduced pressure and refluxing ratio of 0.5-5 the acetic acid whose mass fraction is greater than 95.0% can be obtained.
Owner:BEIJING UNIV OF CHEM TECH
Process for separating acetonitrile-water azeotrope system by adopting ionic liquid extraction distillation
ActiveCN103922963AIncrease relative volatilityGood miscibilityExtractive distillationCarboxylic acid nitrile purification/separationTriflic acidSolvent
Owner:HEBEI UNIV OF TECH
Method and device for purifying silicon tetrachloride of optical fiber grade through total reflux distillation
InactiveCN105502409ARaise the boiling point differenceIncrease relative volatilityHalogenated silanesRefluxTower
The invention discloses a method for purifying silicon tetrachloride of optical fiber grade through total reflux distillation. The method comprises the following steps: after being preheated, silicon tetrachloride waste liquid enters a middle feed opening of a low boiling fraction removal rectifying tower, top steam of the low boiling fraction removal rectifying tower constantly accumulates in a reflux tank after being condensed by a condenser after rectification begins, and the total reflux operation is maintained, so that light components are constantly concentrated at the top of the low boiling fraction removal rectifying tower; when liquid in the reflux tank exceeds the operation liquid level, liquid above an overflow plate in the reflux tank flows into an extraction area to be extracted; tower bottoms, formed by silicon tetrachloride and phosphorus trichloride, of the low boiling fraction removal rectifying tower are discharged from the bottom of the low boiling fraction removal rectifying tower and pumped into a middle feed opening of a silicon tetrachloride purifying tower by a silicon tetrachloride feed pump; overhead fraction of the silicon tetrachloride purifying tower partially refluxes after being condensed by an overhead condenser of the silicon tetrachloride purifying tower, and the other part of overhead fraction is extracted, so as to obtain the silicon tetrachloride product of optical fiber grade. According to the method and the device, after compression, the relative volatility of trichlorosilane and silicon tetrachloride is improved, so that separation becomes easier.
Owner:TIANJIN UNIV
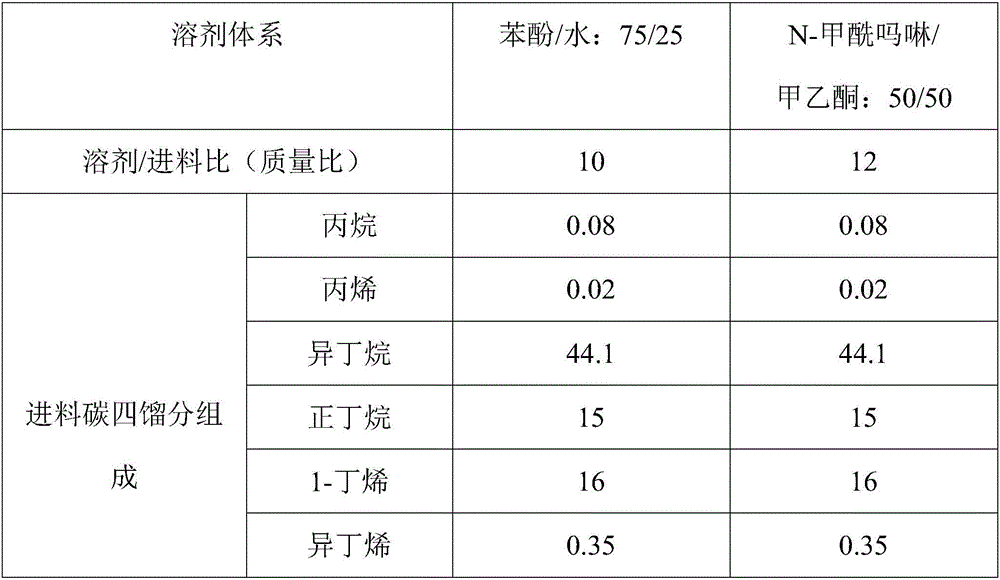
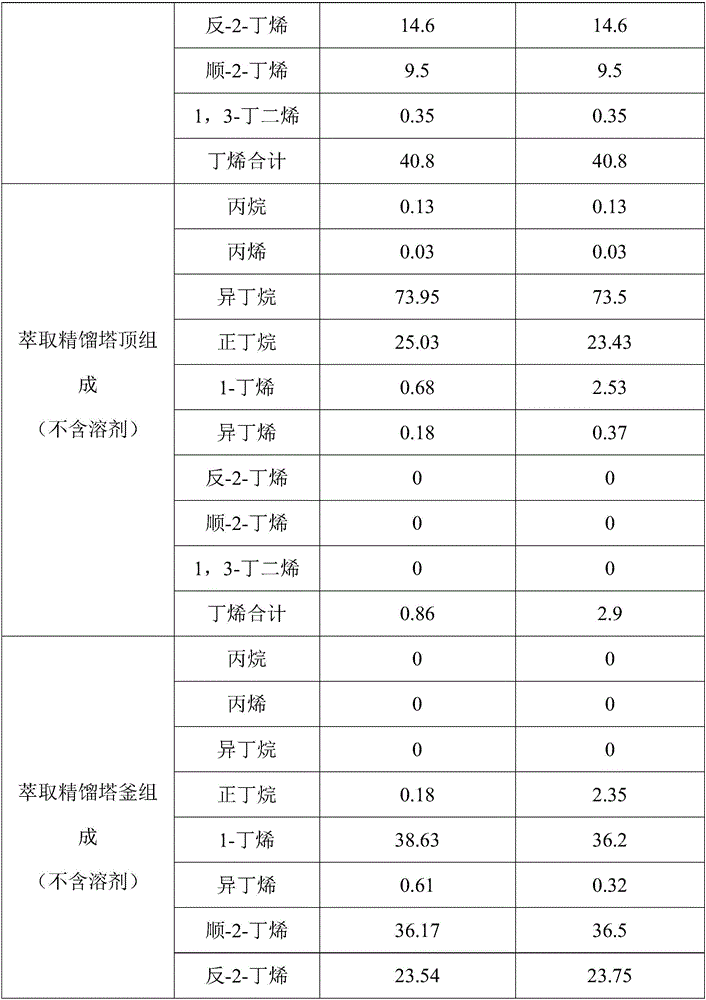
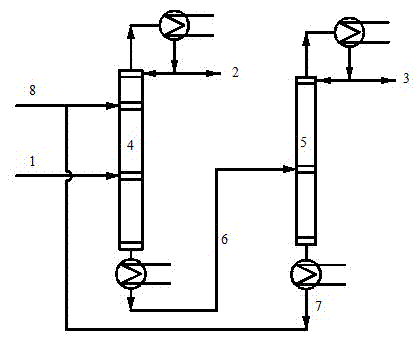
Device and process for separating C4 fractions by adopting binary mixed solvent
InactiveCN106478341ALower gradeHigh purityChemical industryDistillation purification/separationButeneSolvent
The invention provides a device and process for separating C4 fractions by adopting a binary mixed solvent. The device comprises an extraction and rectifying column, an extraction and rectifying column top condenser, an extraction and rectifying column refluxing tank, an extraction and rectifying column refluxing pump, an extraction and rectifying column bottom re-boiler, an extraction and rectifying column residue pump, a stripping column, a stripping column top condenser, a stripping column refluxing tank, a stripping column refluxing pump, a stripping column bottom re-boiler, a stripping column residue pump and a lean solvent cooler. C4 separation is carried out by adopting the process so that the relative volatility between butene and butane can be greatly improved and the solvent selectivity is improved; a solvent / feeding ratio is reduced and the temperature of a column reactor is reduced; the heat load of the column reactor is reduced and the aims of saving energy and reducing consumption are realized.
Owner:DALIAN UNIV OF TECH
Solvent for separating carboxylic acid mixtures by extraction and rectification
InactiveCN103274931AObvious salt effectIncrease relative volatilityOrganic compound preparationCarboxylic acid esters preparationCarboxylic acidSolvent
The invention discloses a solvent for separating carboxylic acid mixtures by extraction and rectification. The solvent is mainly composed of ionic liquid. The solvent can be absolute ionic liquid and also can be a mixture of ionic liquid and preparation materials of cations and anions thereof. The cations of ionic liquid are organic amine. The anions are carboxyl groups. The solvent for extracting, rectifying and separating carboxylic acid mixtures can raise the relative volatilities among components, can raise the separation efficiency, and can reduce the production cost.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA) +1
Composite extractant for separating azeotrope of acetone methanol azeotrope and use method thereof
InactiveCN102190557AEasy to separateStrong salt effect abilityOrganic compound preparationHydroxy compound preparationSteam pressureSolvent
The invention relates to a composite extractant for separating an azeotrope of acetone and methanol and a use method thereof, belonging to the technical field of separation utilization of industrial waste materials. The composite extractant consists of a main extractant and an additive, wherein the additive is a high-valence cation ionic salt, a compound salt or an ionic liquid. According to the invention, an extraction and rectification tower and a solvent recovery tower are used so as to separate a mixture of acetone and methanol; through introducing the salt additive, the separation capacity of the extractant is effectively improved, and the relative volatilities of the acetone and methanol are increased, thus a high-recovery product is obtained, above 95% acetone is obtained by virtue of primary extraction and rectification of the mixture of acetone and methanol with 40-70wt% acetone, the recovery of the acetone is above 85%, and the concentration of methanol is above 95%; and simultaneously, the salt additive has an extremely-low steam pressure and almost non-volatility, which is convenient for recovery and recycling of the composite extractant.
Owner:JILIN UNIV
Batch extractive distillation separation method of isopropyl ether-isopropanol mixture
InactiveCN103159598AIncrease relative volatilityAvoid the problem of poor solubilityEther separation/purificationSolubilityExtractive distillation
The invention discloses a batch extractive distillation separation method of isopropyl ether-isopropanol mixture, and relates to a batch extractive distillation method of isopropyl ether. In the method, an extractive distillation tower, a heating kettle at the bottom of the extractive distillation tower, a condensing unit connected to the top of the extractive distillation tower and a distillation device composed of a receiving tank are adopted. The technological process of the method is as follows: adding raw materials at normal pressure in one step; and continuously adding ethylene glycol monomethyl ether serving as an extraction agent, performing batch operation of the extractive distillation tower, controlling the temperature of the top of the tower and a reflux ratio, and sequentially extracting isopropyl ether and isopropanol. The batch extractive distillation separation method has the advantages that by taking ethylene glycol monomethyl ether as an extraction agent, relative volatility of an isopropyl ether-isopropanol is improved, the problem of poor solubility when ethanediol is taken as the extraction agent, the operation is convenient and easy to control, the cyclic utilization of a solvent is realized, the energy consumption is reduced, the cost is reduced and the separation effect is good, so that the method has good economy and is favorable for environmental friendliness.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Method and special device for separating trimethyl borate from carbinol mixture
ActiveCN102816178AEasy to recycleOvercoming the disadvantages of difficult circulationOrganic compound preparationHydroxy compound preparationSolventTower
The invention discloses a method and a special device for separating trimethyl borate from a carbinol mixture. The method is a salt-adding extraction and rectification method which comprises the following steps of: charging a salt-containing solvent, trimethyl borate and carbinol mixture from the upper part and middle part of an extraction and rectification tower respectively, carrying out salt-adding extraction and rectification, withdrawing pure trimethyl borate from the top of the tower, and obtaining the mixture of carbinol, salt and solvent at a tower kettle; and charging the mixture of carbinol, salt and solvent into a solvent recovering tower from the middle, wherein the distillate on the top of a recovery tower is the carbinol, obtaining the salt-containing solvent at the tower kettle, and repeatedly charging the salt-containing solvent into the extraction and rectification tower to be used. The method is better in effect due to the salt-adding extractant compared with the extractant, has the advantages of being convenient to recycle and easy to realize on the industry, can be used for overcoming the defect that the sold salt in a salt-adding rectification technology is hard to cycle, can be used for greatly improving the separation effect since the relative volatility of the trimethyl borate and the carbinol is increased, has the advantages of being low in equipment investment and low in energy consumption, and can be used for the industrial application for the separation and purification of the trimethyl borate.
Owner:江苏宏梓新能源科技有限公司
Intermittent extractive distillation and separation method for isopropyl ether-isopropanol mixture
InactiveCN103044215AIncrease relative volatilityAvoid the problem of poor solubilityEther separation/purificationOrganic compound preparationSolubilityReflux
The invention provides an intermittent extractive distillation and separation method for an isopropyl ether-isopropanol mixture and relates to a distillation and separation method. A device used by the method comprises an extractive distillation tower, a heating kettle and a distillation device, wherein the heating kettle is arranged at the bottom of the extractive distillation tower; and the distillation device is connected to the top of the extractive distillation tower and comprises a condensing device and a receiving tank. The technical process comprises the following steps: adding raw materials at a normal pressure at one time, continuously adding ethylene glycol monomethyl ether to the raw materials as an extracting agent, carrying out intermittent operation on the raw materials by using the extractive distillation tower, controlling a temperature and a reflux ratio at the top of the tower, and extracting isopropyl ether and isopropanol in sequence. The intermittent extractive distillation and separation method for the isopropyl ether-isopropanol mixture, provided by the invention, has the advantages as follows: through using the ethylene glycol monomethyl ether as the extracting agent, the relative volatility of an isopropyl ether-isopropanol system is enhanced, the problem that the solubility is poor when the traditional ethylene glycol is used as the extracting agent is avoided, convenient operation and easy control are achieved, solvents are recycled, energy consumption is reduced, the cost is reduced, a better separating effect and very good economic efficiency are obtained, and the intermittent extractive distillation and separation method is beneficial for environmental protection.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
High pure hexafluoropropylene oxide preparation method using extraction rectification
ActiveCN101455908AReduce volatilityIncrease relative volatilityOrganic chemistryExtractive distillationMass ratioTower
The invention relates to a method for preparing high-purity hexafluoropropylene oxide by extraction and rectification. The method comprises the following steps: selecting epoxy chloropropane as an extracting agent, wherein the mass ratio of the extracting agent to HFPO-HFP mixture feed is controlled between (1-200): 1; introducing the HFPO-HFP mixture into an extraction rectifying tower, spraying the epoxy chloropropane from the tower top, and separating HFPO from the tower top to obtain HFPO with purity over 99 percent; and discharging HFP and the epoxy chloropropane from the bottom of the extraction rectifying tower to be pressed into a reclaiming tower once again, separating the HFP from the top of the reclaiming tower, and discharging the epoxy chloropropane from the bottom of the reclaiming tower to be pumped once again to return a spraying port on the top of the extraction rectifying tower for cycle use. The method selects the epoxy chloropropane as the extracting agent to make relative volatility of the HFPO and the HFP reach 1.5 to 3.7, so that the HFPO and the HFP can be well separated; meanwhile, the number of column plates required for rectification can be reduced, and investment cost can be reduced.
Owner:SHANDONG DONGYUE WEILAI HYDROGEN ENERGY MATERIAL CO LTD
Mixed solvent for extractive distillation and separation of C4
InactiveCN102344330AIncrease relative volatilityReduce the number of boardsDistillation purification/separationInorganic saltsExtractive distillation
The invention provides a mixed solvent which can improve relative volatility among each ingredient of C4 and improve solvent selectivity, manly comprising N-methyl pyrrolidone, inorganic salt and water. Using the mixed solvent disclosed herein can greatly reduce the solvent ratio, reduce the cost, reduce the temperature of the column bottom, and save material consumption and energy consumption.
Owner:CHINA PETROLEUM & CHEM CORP +1
New technology for separating isopropanol-isopropyl ether azeotrope by using ionic liquids
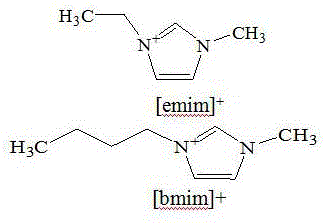
InactiveCN105111046AHigh purityEasy to separateEther separation/purificationOrganic compound preparationSolubilityReflux
The invention relates to a rectification separation method, and concretely relates to a new technology for separating isopropanol-isopropyl ether azeotrope by using ionic liquids. The new ionic liquids [emim]<+>[BF4]<-> and [bmim]<+>[BF4]<-> (with the structures shown in figure 1) are adopted in the invention as extractants, and the isopropanol-isopropyl ether azeotrope is separated through adotpign a continuous rectification method. Rectifying towers adopted in the technology comprise an extraction rectifying tower, a solvent recovery tower and other auxiliary devices. The technology comprises the following steps: adding the isopropanol-isopropyl ether azeotrope into the central portion of the extraction rectifying tower, recovering the ionic liquids in the solvent recovery tower, pumping the ionic liquids into the extraction rectifying tower through using a pump in order to realize cycle use, and controlling different temperatures and reflux ratios of the above two towers in order to realize continuous production. The technology breaks use of a traditional solvent glycol as an extractant, improves the relative volatility of isopropanol-isopropyl ether, avoids the problem of poor solubility of traditional glycol as the extractant, and has the advantages of convenient operation, large operation elasticity and continuous production.
Owner:UNIV OF JINAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com