Batch extractive distillation separation method of isopropyl ether-isopropanol mixture
A technology of extractive distillation and separation method, which is applied in the field of separation of isopropyl ether-isopropanol mixture, which can solve the problems of non-separation and difficulty in obtaining pure isopropyl ether, and achieve easy control, environmental protection and separation effect Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Raw materials to be separated: isopropyl ether (99.9%), isopropanol (99.9%)
[0020] Extractant: Ethylene glycol methyl ether (99.8%)
[0021] Batch extractive distillation tower (packed tower): the separation process is as follows:
[0022] Tower inner diameter: Φ40mm (built-in θ4×4 mesh ring packing) solvent ratio: 3:1
[0023] Reflux ratio: 1~4
[0024] Taft kettle temperature: 60~125℃
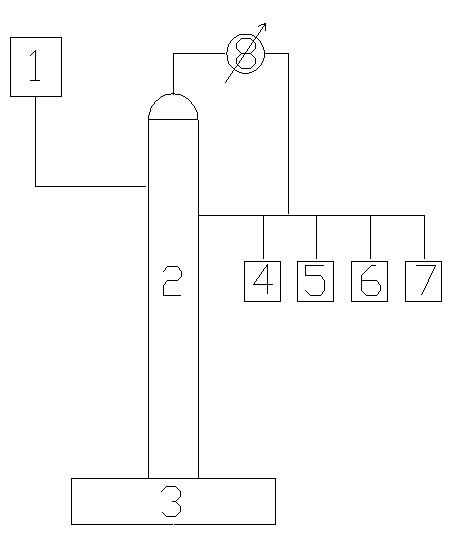
[0025] by figure 1 The shown flow device adopts intermittent operation to separate the mixture of isopropyl ether and isopropanol. The specific process is as follows: the diameter of the extractive distillation tower used is Φ40mm, and theta mesh ring packing is installed inside. The packing layer is 2000mm. At 150mm, add 1500ml of isopropanol-isopropyl ether mixture (including 83.7% isopropyl ether and 16.3% isopropyl ether, both in mass fraction) to the kettle of the heating tower, add the extractant ethylene glycol methyl ether to the High slot. Turn on the power supply of t...
Embodiment 2
[0027] Raw materials to be separated: isopropyl ether (99.9%), isopropanol (99.9%)
[0028] Extractant: Ethylene glycol methyl ether (99.8%)
[0029] Batch extractive distillation tower (packed tower): the separation process is as follows:
[0030] Tower inner diameter: Φ40mm (built-in θ4×4 mesh ring packing)
[0031] Solvent ratio: 3:1
[0032] Reflux ratio: 1~4
[0033] Taft kettle temperature: 60~125℃
[0034] by figure 1The shown flow device adopts intermittent operation to separate the mixture of isopropyl ether and isopropanol. The specific process is as follows: the diameter of the extractive distillation tower used is Φ40mm, and theta mesh ring packing is installed inside. The packing layer is 2000mm. At 150mm, add 1500ml of isopropanol-isopropyl ether mixture (including 85.5% isopropyl ether and 14.5% isopropyl ether, both of which are mass fractions) in the heating tower kettle, add the extractant ethylene glycol methyl ether to the High slot. Turn on the powe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com