Reaction-rectification-separation-refinement novel method, technique and device of ethylene glycol and 1,2-butanediol
A separation method and technology of a reactive distillation column are applied in the field of processes and devices, separation methods of ethylene glycol and 1,2-butanediol, and can solve the problems of affecting the yield of ethylene glycol, unfavorable energy saving, poor recovery and the like , to achieve the effect of high quality recycled products, saving equipment investment, and low relative volatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
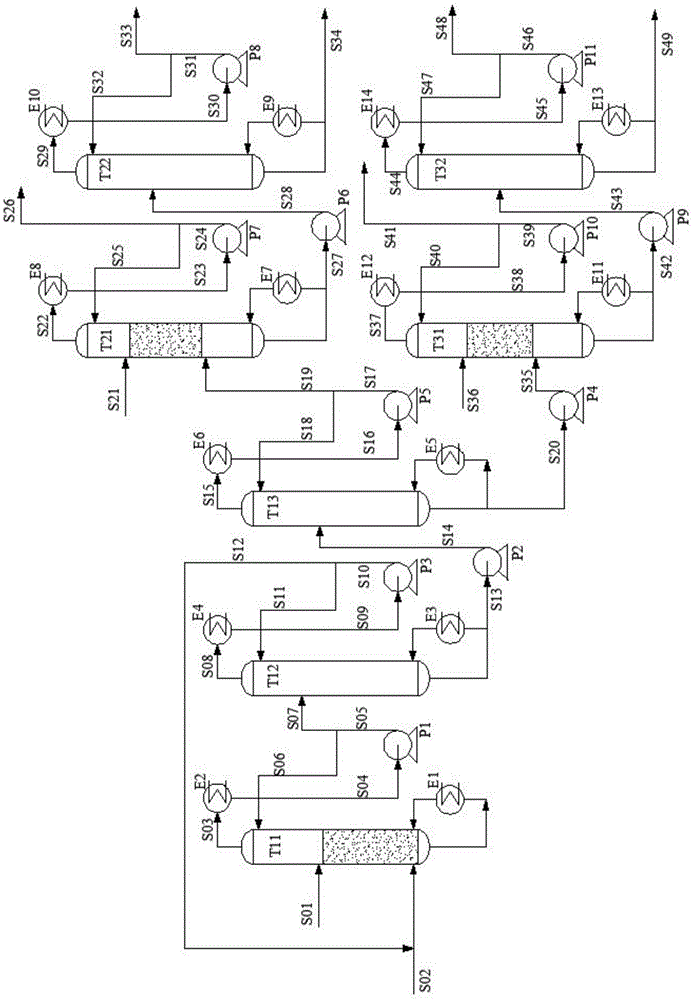
[0066] The method of the present invention is used for ethylene glycol and 1,2-butanediol mixture reactive distillation separation process, such as figure 1 As shown, including acetal reactive distillation column (T11), aldehyde recovery column (T12), acetal product separation column (T13), acetal product hydrolysis distillation column (T21, T31), ethylene glycol purification column (T22 ), 1,2-butanediol purification column (T32), condenser, reboiler, pump and related feed lines and lines connecting the above equipment. The diol mixed raw material S01 (the molar ratio of ethylene glycol and 1,2-butanediol: 0.6:0.4) enters the reactive distillation column (T11) with 21 theoretical plates from the sixth theoretical plate, and the reactant acetaldehyde is removed from the column. The kettle enters the reactive rectification tower, the total amount of reactants and the molar ratio of diol are 1.19:1, and the reaction section is set below the diol feed position to the tower kettle...
Embodiment 2
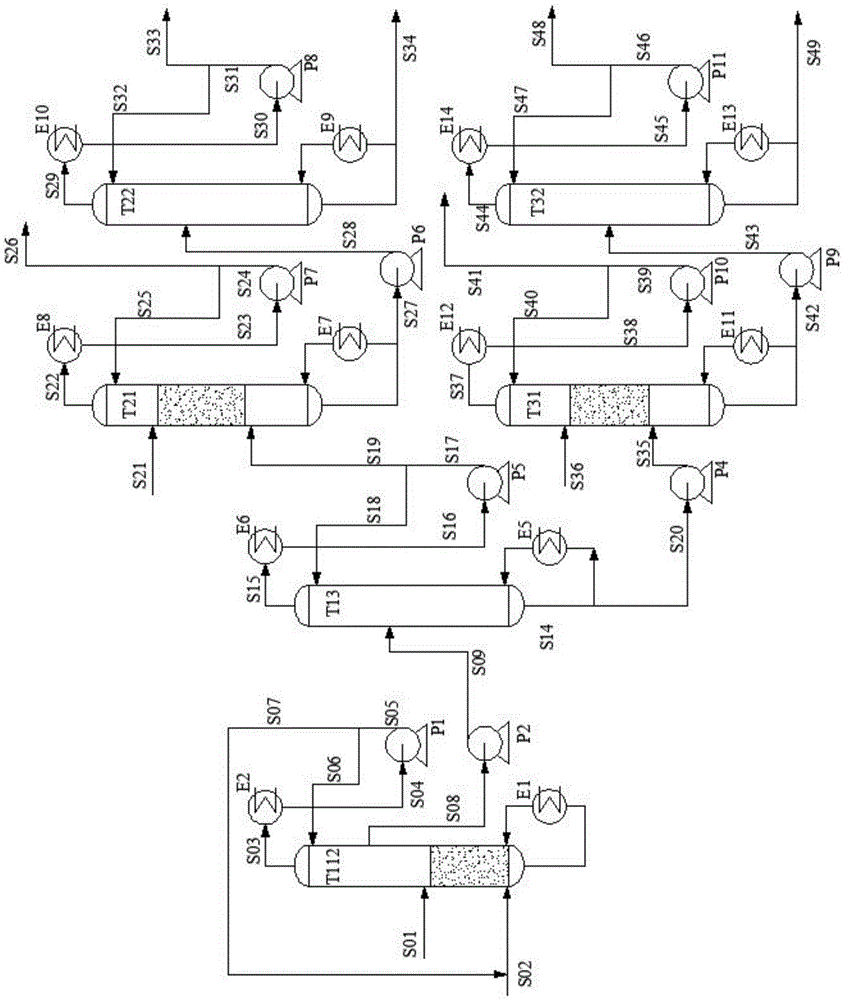
[0069] The method of the present invention is used for ethylene glycol and 1,2-butanediol mixture reactive distillation separation process, such as figure 2 As shown, it is the improvement process of embodiment 1, and the acetal reactive rectifying tower (T11) and the acetaldehyde recovery tower (T12) in the original process are merged into the acetal reactive rectifying tower (T112), including the acetal reactive rectifying tower (T112). Distillation column (T112), acetal product separation column (T13), acetal product hydrolysis and rectification column (T21, T31), ethylene glycol purification column (T22), 1,2-butanediol purification column (T32), Condenser, reboiler, pump and associated feed lines and lines connecting the above equipment. The diol mixed raw material S01 (the molar ratio of ethylene glycol and 1,2-butanediol: 0.6:0.4) enters the reactive distillation column (T112) with 30 theoretical plates from the 15th theoretical plate, and the reactant acetaldehyde is ...
Embodiment 3
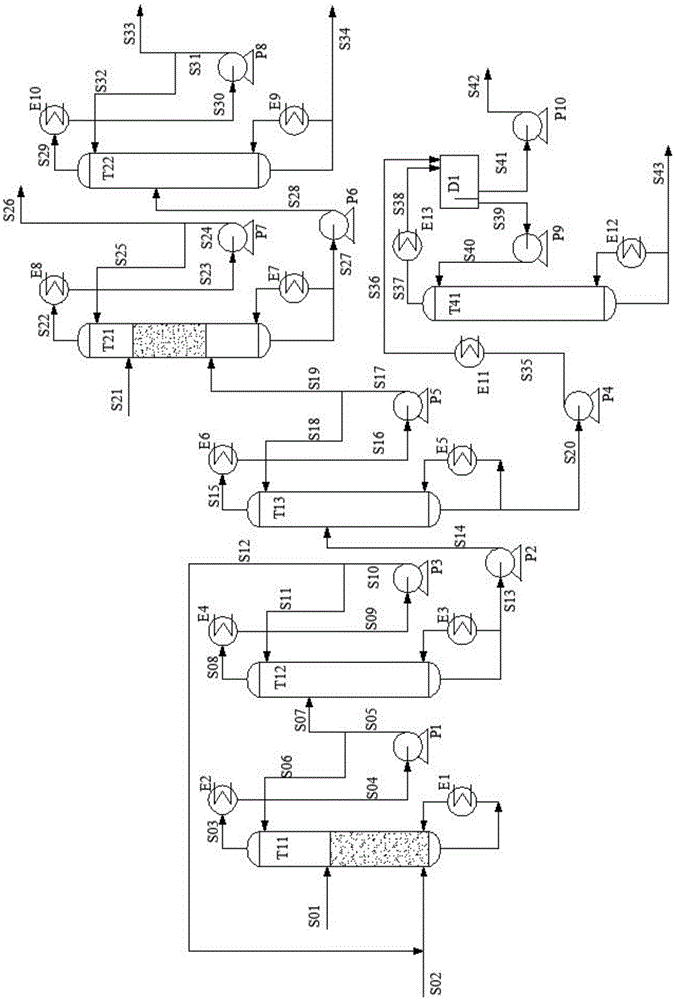
[0072] The method of the present invention is used for ethylene glycol and 1,2-butanediol mixture reactive distillation separation process, such as figure 2 As shown, for the improved process of Example 1, it is considered to separate the acetal product of 1,2-butanediol as a product without hydrolysis, including the acetal reactive distillation column (T11) and the aldehyde recovery column (T12) , acetal product separation tower (T13), ethylene glycol acetal product hydrolysis and rectification tower (T21), ethylene glycol purification tower (T22), 1,2-butanediol acetal product purification tower (T41), Phaser (D1), condenser, reboiler, pump and associated feed lines and lines connecting the above equipment. The other operations of the third embodiment are the same as those of the first embodiment. The extracted stream S20 of the acetal product separation tower (T13) is cooled to 65°C and then enters the phase separator D1, phase-separated at 1 atm and 65°C, the water phase...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com