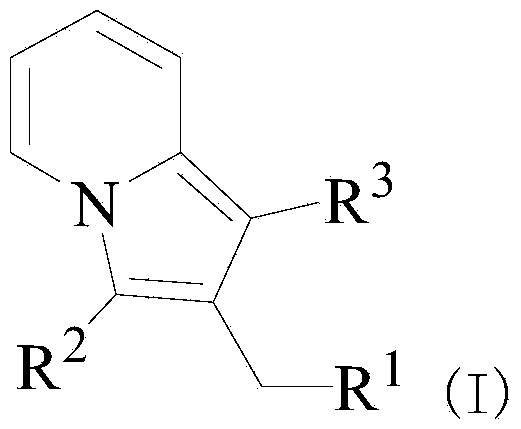
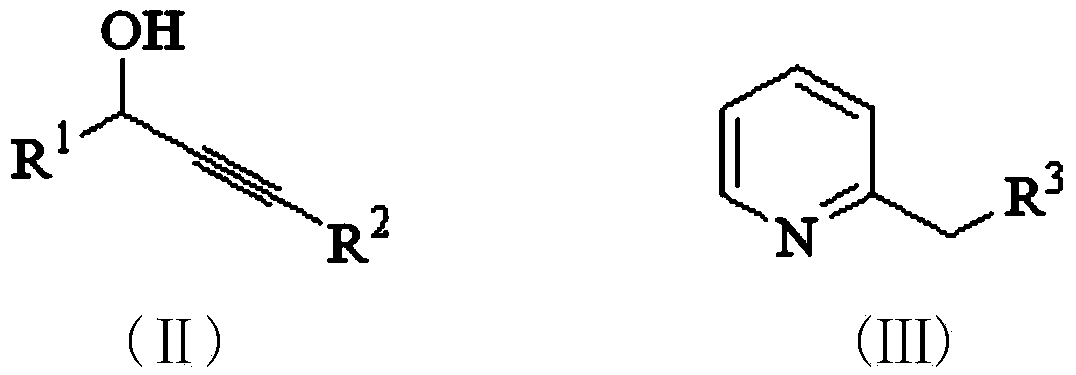Purrocoline derivative and synthetic method and application thereof
A synthesis method and compound technology, which can be used in pharmaceutical combinations, medical preparations containing active ingredients, pharmaceutical formulations, etc., can solve the problems of large chemical pollution, increase reaction consumption, etc., and achieve simplified operation, good activity, and reduced chemical pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
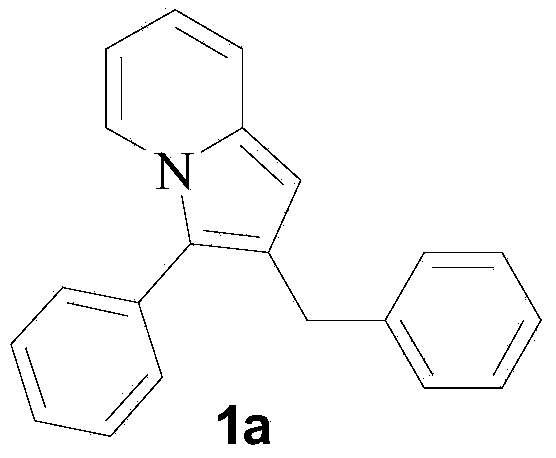
[0025] Embodiment 1: the preparation of 2-benzyl-3-phenyl indolizine
[0026] 0.3 mmol (62.4 mg) of 1,3-diphenyl-prop-2-yn-1-ol and 1.5 mmol (139.5 mg) of 2-picoline were added to the flask, followed by 1,3-diphenyl- Prop-2-yn-1-ol was reacted with 20 mol% (30 mg) of samarium trifluoromethanesulfonate catalyst at 120°C and stirred until complete (TLC followed the reaction, about 24 hours). After the reaction is complete, cool to room temperature, pour the reactant into 10-30mL of water, and add 20-30mL of ethyl acetate for extraction, collect the extract phase, wash with saturated brine (10-20mL) three times, and then wash with anhydrous Na 2 SO 4 Dry, filter, remove the solvent from the filtrate under reduced pressure, and purify the residue by silica gel column chromatography, eluting with an eluent composed of ethyl acetate and petroleum ether with a volume ratio of 1:20, and evaporate the eluent under reduced pressure solvent to obtain 76.4 mg of green oily liquid 1a.
...
Embodiment 2
[0031] Embodiment 2: Preparation of 2-benzyl-1-methyl-3-phenylindolizine
[0032] 0.3 mmol (62.4 mg) of 1,3-diphenyl-prop-2-yn-1-ol and 1.5 mmol (160 mg) of 2-ethylpyridine were added to the flask, followed by 1,3-diphenyl-prop-2-yn-1-ol -2-Alkyn-1-ol was reacted with 20 mol% (30 mg) of samarium trifluoromethanesulfonate as a catalyst at 120°C until complete (TLC followed the reaction, about 24 hours). After the reaction is complete, cool to room temperature, pour the reactant into 10-30 mL of water, extract with 20-30 mL of ethyl acetate, collect the extract phase, wash with saturated brine (10-20 mL) three times, and finally wash with anhydrous Na 2 SO 4 Dry, filter, remove the solvent from the filtrate under reduced pressure, and purify the residue by silica gel column chromatography, eluting with an eluent composed of ethyl acetate and petroleum ether with a volume ratio of 1:20, and evaporate the eluent under reduced pressure solvent to obtain 80.2 mg of green oily liqu...
Embodiment 3
[0037] Embodiment 3: Preparation of 2-benzyl-3-phenylindolizine-1-carboxylate ethyl ester
[0038]0.3 mmol (62.4 mg) of 1,3-diphenyl-prop-2-yn-1-ol and 1.5 mmol (247.5 mg) of 2-acetoxyethylpyridine were added to the flask, followed by 1,3-diphenyl 20 mol% (30 mg) of samarium trifluoromethanesulfonate catalyst was used in the base-prop-2-yn-1-ol, and the reaction was stirred at 120°C until complete (TLC followed the reaction, about 24h). After the reaction is complete, cool to room temperature, pour the reactant into 10-30mL of water, and extract with 20-30mL of ethyl acetate, collect the extract phase, wash with saturated brine (10-20mL) three times, and finally wash with anhydrous Na 2 SO 4 Dry, filter, remove the solvent from the filtrate under reduced pressure, and purify the residue by silica gel column chromatography, eluting with an eluent composed of ethyl acetate and petroleum ether with a volume ratio of 1:20, and evaporate the eluent under reduced pressure solvent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com