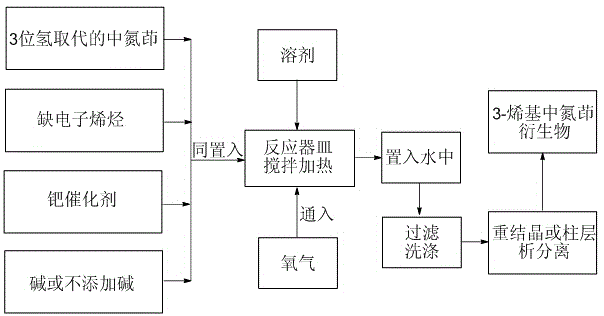
Green preparation method for 3-alkenyl indolizine derivative
An indene derivative and alkenyl technology is applied in the field of organic synthetic chemistry, and can solve the problems of rising raw material cost, environmental hazards, complicated process flow, etc., and achieve the effects of reducing synthesis cost, simple process flow, and cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] as attached figure 1 process flow, take 1- N , N -Dimethylcarbamido-indolizine is 38.0 mg (equivalent to 0.20 mmol), butyl acrylate is 57 microliters (equivalent to 0.40 mmol), palladium acetate is 2.2 mg (equivalent to 0.010 mmol), acetic acid 10.0 mg of potassium (equivalent to 0.10 mmol) and 2.0 ml of dimethyl sulfoxide were heated and stirred at 100 degrees Celsius for 5 hours under 1 atmospheric pressure of oxygen, and 56.0 mg of the target product 3-alkenyl indolizine derivative of Example 1 was isolated (The yield is 89%).
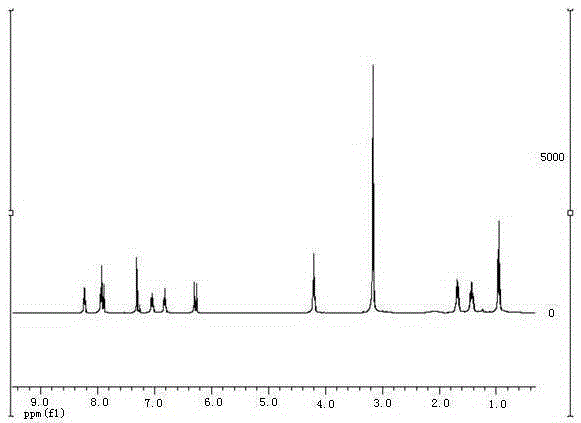
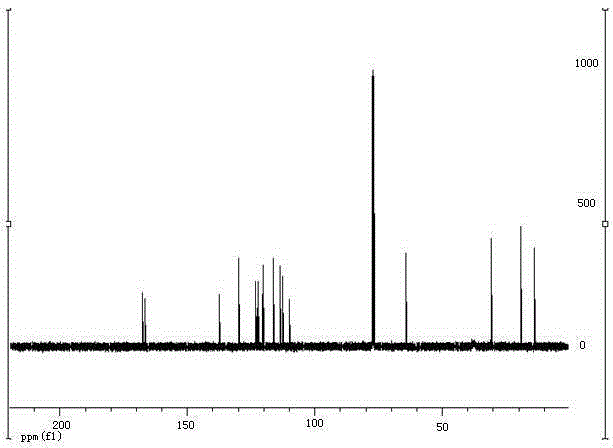
[0037] The target product in Example 1 was analyzed by nuclear magnetic resonance spectrometer (model: AVANCE400MHz, manufacturer: Bruker, Switzerland) to obtain figure 2 The H NMR spectra shown and image 3 The carbon NMR spectrum shown. The parameters of the former are 1 HNMR (CDCl 3 ,400MHz):8.22(d, J =7.0Hz,1H),7.94(d, J =8.2Hz,1H),7.91(d, J =15.4Hz,1H),7.31(s,1H),7.04(dd, J =8.6,6.9Hz,1H),6.82(t, J =6.6Hz,1H),6.28(d, J =15....
Embodiment 2
[0039] as attached figure 1 The technical process, take 1,2-dicarbonate ethyl-indolizine as 52.2 mg (equivalent to 0.20 mmol), N , N- 51.6 µl (equivalent to 0.50 mmol) of dimethylacrylamide, 1.1 mg (equivalent to 0.0050 mmol) of palladium acetate, 12.0 mg (equivalent to 0.12 mmole) of potassium acetate, 2.0 mL of dimethylsulfoxide Under 1 atmospheric pressure of oxygen, heating and stirring at 100°C for 6 hours, 68.1 mg of the target product of Example 2 was isolated (95% yield).
Embodiment 3
[0041] as attached figure 1 The technological process of taking 2,5-dimethyl-1-cyano-indolizine is 34.0 mg (equivalent to 0.20 mmol), dimethyl maleate is 28.8 mg (equivalent to 0.20 mmol), acetic acid 4.5 mg of palladium (corresponding to 0.02 mmol), 13.5 mg of sodium bicarbonate (corresponding to 0.16 mmol) and 2.0 ml of N,N-dimethylformamide were heated and stirred at 110 degrees Celsius for 16 hours under 1 atmosphere of oxygen, 40.0 mg of the target product of Example 3 was isolated (64% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com