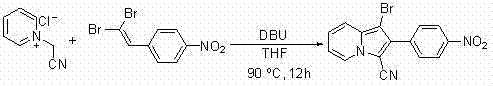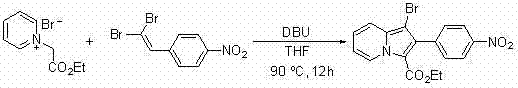A method for synthesizing 1-halogen-2-aryl indolizine compounds
A compound, the technology of pyrazine, which is applied in the field of organic and pharmaceutical synthesis, can solve the problems of few suitable substrates, many synthesis steps, and difficult synthesis, and achieve the effects of mild reaction conditions, high reaction efficiency, and wide substrate range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the synthesis of 1-bromo-2-p-nitrophenyl-3-ethoxycarbonyl indolizine
[0054]
[0055] Add 0.3mmol N-ethoxycarbonylmethylpyridinium bromide, 0.6mmol 1,1-dibromo-2-p-nitrophenylethylene, 5mL tetrahydrofuran, 1.35mmol DBU to a 25mL round bottom flask, and then the reaction solution Magnetically stirred and refluxed at 90°C for 12 hours. After the reaction was completed, cool to room temperature and evaporate the solvent under reduced pressure. The residue was subjected to column chromatography with ethyl acetate / petroleum ether=1:10 (v / v) as the eluent. The desired product can be obtained by separation and purification. The product is a yellow solid with a yield of 95%.
[0056] 1 H NMR (400MHz, CDCl 3 ): δ = 9.60(d, J = 7.2 Hz, 1H), 8.37(d, J = 8.4 Hz, 2H),7.73(d, J = 8.0 Hz, 2H), 7.53(d, J = 9.6 Hz, 1H), 7.16(t, J = 7.8 Hz, 1H), 6.93(t, J = 7.0 Hz, 1H), 4.50(q, J = 7.0, 7.2Hz, 2H), 1.50(t, J = 7.0 Hz, 3H).
Embodiment 2
[0057] Embodiment 2: Synthesis of 1-bromo-2-p-fluorophenyl-3-ethoxycarbonyl indolizine
[0058]
[0059] Add 0.3mmol N-ethoxycarbonylmethylpyridinium bromide, 0.6mmol 1,1-dibromo-2-p-fluorophenylethylene, 5mL tetrahydrofuran, 1.35mmol DBU to a 25mL round bottom flask, and then the reaction solution was Magnetic stirring and reflux reaction at 90°C for 12 hours, after the completion of the reaction, cool to room temperature, evaporate the solvent under reduced pressure, and use ethyl acetate / petroleum ether=1:10 (v / v) as the eluent for column chromatography to separate the residue The desired product can be obtained after purification. The product is a yellow-green solid with a yield of 63%.
[0060] 1 H NMR (400MHz, CDCl 3 ): δ = 9.45 (d, J = 7.2 Hz, 1H), 7.40-7.35 (m, 3H), 7.10 (t, J = 8.4Hz, 2H), 6.97(t, J = 7.8 Hz, 1H), 6.76(t, J = 7.0 Hz, 1H), 4.39(q, J = 7.2, 6.8 Hz, 2H), 1.39(t, J = 7.2 Hz, 3H).
Embodiment 3
[0061]Embodiment 3: Synthesis of 1-bromo-2-p-chlorophenyl-3-ethoxycarbonyl indolizine
[0062]
[0063] Add 0.3mmol N-ethoxycarbonylmethylpyridinium bromide, 0.6mmol 1,1-dibromo-2-p-chlorophenylethylene, 5mL tetrahydrofuran, 1.35mmol DBU to a 25mL round bottom flask, and then the reaction solution was Magnetic stirring and reflux reaction at 90°C for 12 hours, after the completion of the reaction, cool to room temperature, evaporate the solvent under reduced pressure, and use ethyl acetate / petroleum ether=1:10 (v / v) as the eluent for column chromatography to separate the residue The desired product can be obtained after purification. The product is light yellow solid with a yield of 85%.
[0064] 1 H NMR (400MHz, CDCl 3 ): δ=9.46(d, J = 7.2 Hz, 1H), 7.40-7.35(m, 5H), 6.98(t, J = 7.8 Hz, 1H), 6.77(t, J = 6.8 Hz, 1H), 4.39(q, J = 7.0, 7.2 Hz, 2H), 1.40(t, J =7.2 Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com