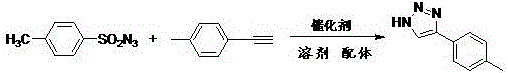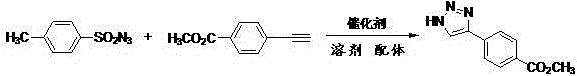A kind of synthetic method of nh-1,2,3-triazole compound
A technology of triazole compound and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of high reaction temperature, many synthesis steps, and long reaction time, and achieve the effects of mild reaction conditions, high reaction efficiency, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Synthesis of 4-phenyl-1H-1,2,3-triazole:
[0041] The reaction formula is:
[0042]
[0043] The specific steps are: add 0.3mmol p-toluenesulfonyl azide, 0.33mmol phenylacetylene, 0.03mmol copper acetate, 0.015mmol o-aminophenol, and 3mL acetonitrile into a 25mL round-bottomed flask, and stir the reaction at 25°C for 20 minutes. Add 1mL of distilled water, then the reaction solution was magnetically stirred and refluxed at 80°C for 12 hours. After the reaction was completed, it was cooled to room temperature, and the reaction solution was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and then reduced pressure The solvent was evaporated to obtain the crude product, which was separated and purified by column chromatography using ethyl acetate / petroleum ether=1:2 (v / v) as the eluent to obtain the desired product. The product is a white solid with a yield of 90%. The proton nuclear magnetic resonan...
Embodiment 2
[0045] Synthesis of 4-phenyl-1H-1,2,3-triazole:
[0046] The reaction formula is:
[0047]
[0048] Add 3mmol p-toluenesulfonyl azide, 3mmol phenylacetylene, 0.3mmol copper acetate, 0.15mmol hydroquinone, 30mL acetonitrile into a 100mL round-bottomed flask, stir and react under magnetic force at 30°C for 30 minutes, then add 5mL distilled water, then The reaction solution was magnetically stirred and refluxed at 130°C for 1 hour. After the reaction was completed, it was cooled to room temperature, and the reaction solution was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain The crude product was separated and purified by column chromatography using ethyl acetate / petroleum ether=1:2 (v / v) as the eluent to obtain the desired product. The product is a white solid with a yield of 88%. The proton nuclear magnetic resonance spectrogram result of ...
Embodiment 3
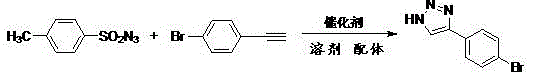
[0050] Synthesis of 4-p-tolyl-1H-1,2,3-triazole:
[0051] The reaction formula is:
[0052]
[0053] The specific steps are: add 0.6mmol p-toluenesulfonyl azide, 1.2mmol p-toluene acetylene, 0.06mmol cuprous acetate, 0.03mmol p-aminophenol, 6mL tetrahydrofuran into a 50mL round bottom flask, and stir the reaction under magnetic force at 25°C After 30 minutes, 2 mL of distilled water was added, and then the reaction solution was magnetically stirred and refluxed for 20 hours at 90°C. After the reaction was completed, it was cooled to room temperature, and the reaction solution was extracted with ethyl acetate. The organic layer was washed with saturated saline, and dried over anhydrous sodium sulfate. Finally, the solvent was evaporated under reduced pressure to obtain the crude product, which was separated and purified by column chromatography using ethyl acetate / petroleum ether=1:2 (v / v) as the eluent to obtain the desired product. The product is a brown-red solid with a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com