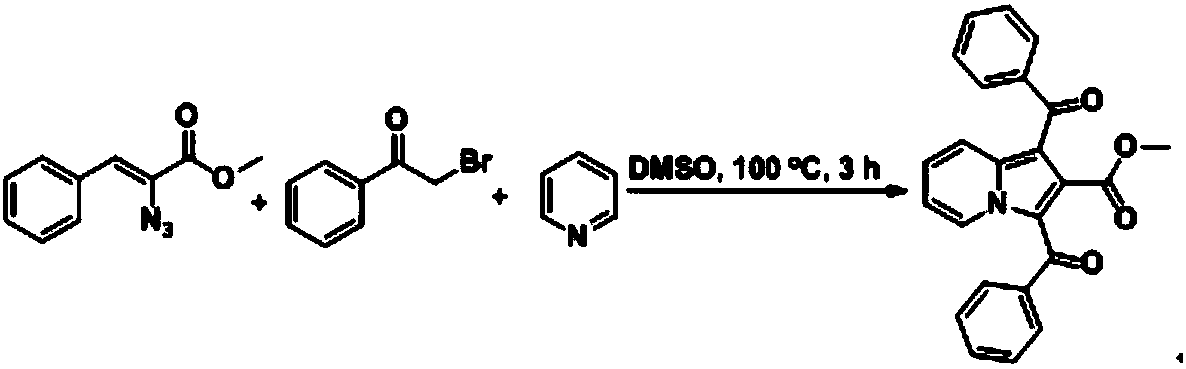1,2,3-trisubstituted indolizine derivative and preparation method thereof
An indolizine derivative and tri-substitution technology, which is applied in the field of 1,2,3-trisubstituted indolizine derivatives and their preparation, can solve the problems of fast forward and reverse reactions, harsh conditions, complex reactions, etc., and achieves energy reduction. The effect of low consumption, mild reaction conditions and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add bromoacetophenone (1.0mmol), methyl azidocinnamate (0.5mmol) and pyridine (0.75mmol), DMSO (3mL) to a 25mL reaction tube, react at 100°C for 3h, TLC (thin layer chromatography method) tracking reaction, after the reaction was finished, extracted with dichloromethane, anhydrous Na 2 SO 4 After drying and column chromatography (developer petroleum ether / ethyl acetate v / v=3:1), a yellow solid A1 can be obtained in a yield of 78%. The reaction principle is as follows.
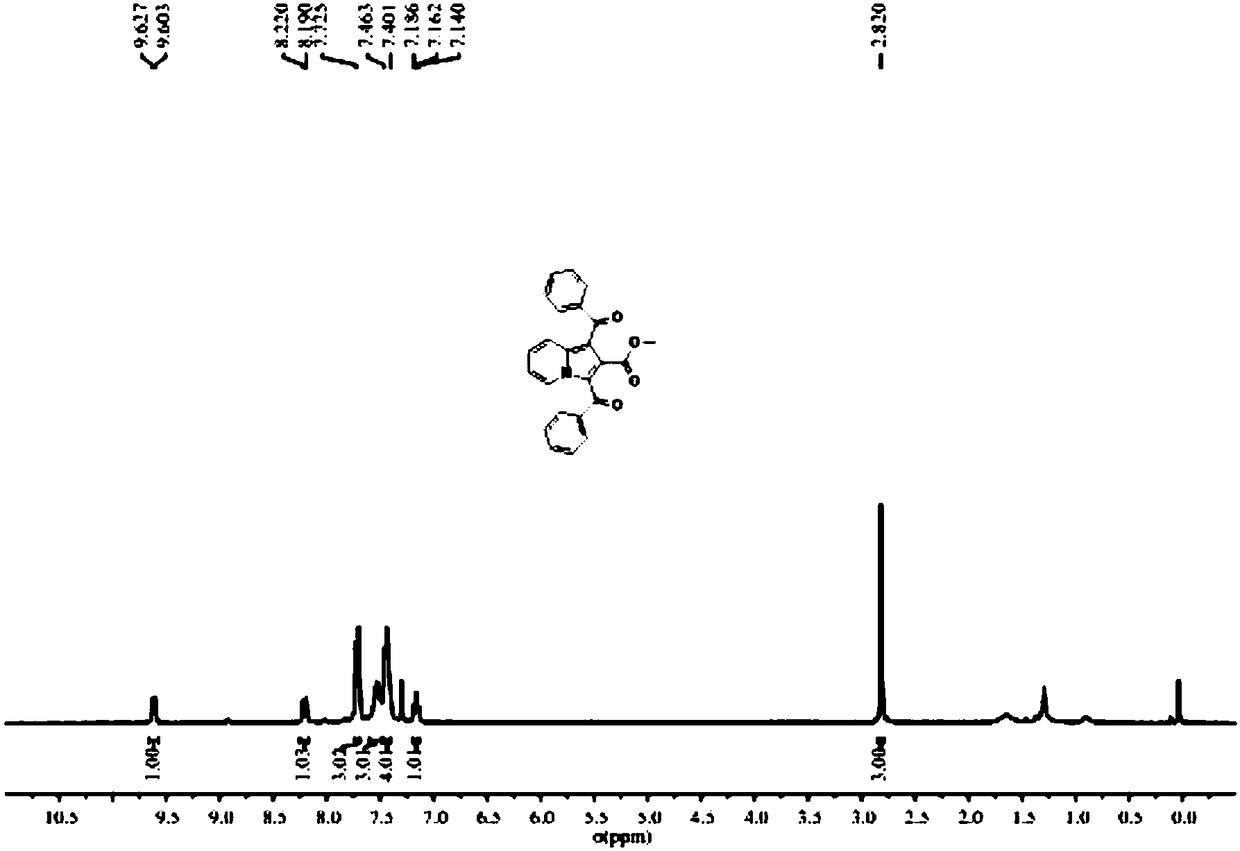
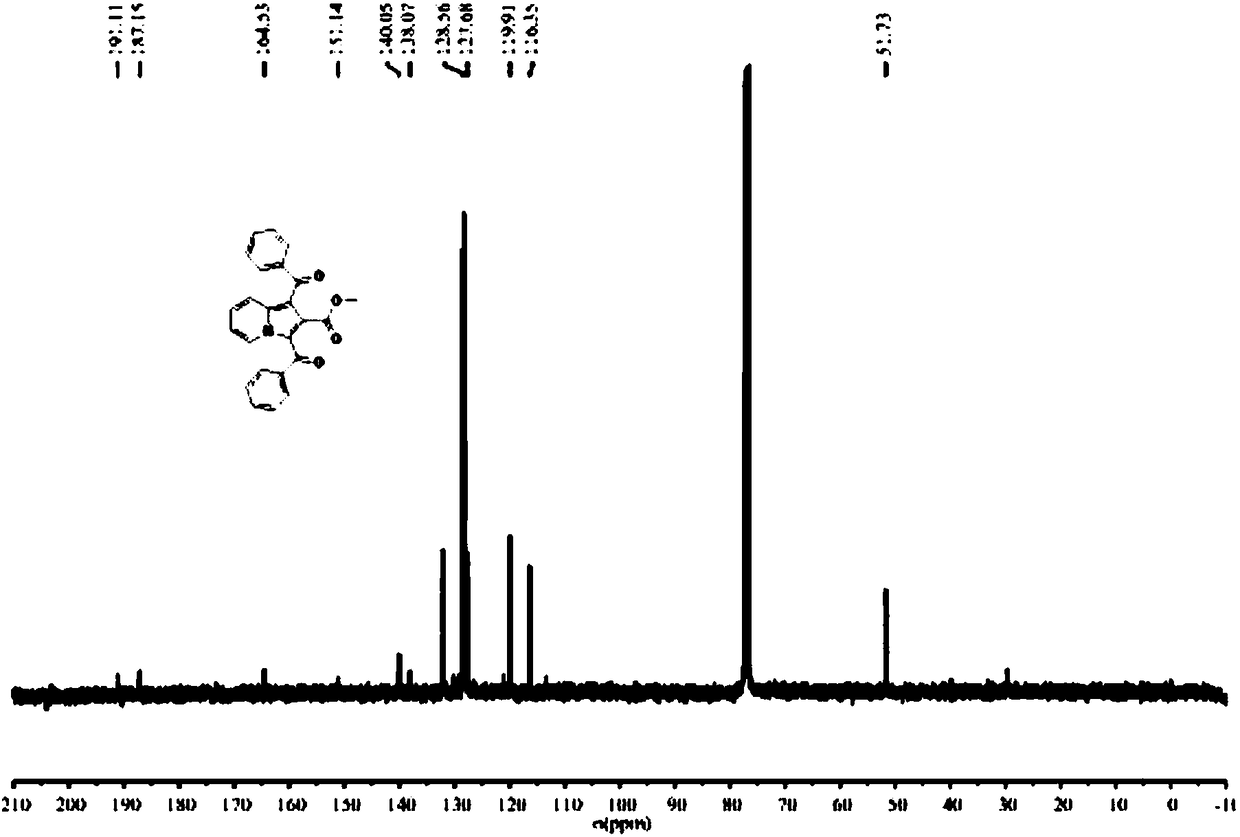
[0035] The H NMR and C NMR spectra of compound A1 correspond to figure 1 , figure 2 , the obtained data are as follows: Methyl1,3-dibenzoylindolizine-2-carboxylate(A1), 1 H NMR (300MHz, CDCl 3 )δ9.61(d, J=7.2Hz, 1H), 8.21(d, J=9.0Hz, 1H), 7.71(d, J=7.2Hz, 3H), 7.56-7.49(m, 3H), 7.46- 7.40(m,4H),7.16(t,J=6.9Hz,1H),2.82(s,3H); 13 C NMR (75MHz, CDCl 3 )δ 191.1, 187.1, 164.5, 151.1, 140.2, 140.0, 138.0, 132.1, 132.0, 128..5, 128.5, 128.3, 127.7, 127.6, 119.9, 116.3, 51.7. It can be seen that the sin...
Embodiment 2
[0038] Add 4-methylbromoacetophenone (1.0mmol), methyl azidocinnamate (0.5mmol) and pyridine (0.75mmol), DMSO (3mL) to a 25mL reaction tube, react at 100°C for 3h, TLC Follow up the reaction, after the reaction is finished, extract with dichloromethane, anhydrous Na 2 SO 4 After drying and column chromatography (developer petroleum ether / ethyl acetate v / v=3:1), a yellow solid A2 can be obtained in a yield of 75%. The reaction principle is as follows.
[0039]
[0040] The results of the H NMR and C NMR spectra of compound A2 are as follows: Methyl1,3-bis(4-methylbenzoyl)indolizine-2-carboxylate(A2), 1 H NMR (300MHz, CDCl 3 )δ9.51(d, J=6.9Hz, 1H), 8.12(d, J=9.0Hz, 1H), 7.64-7.61(m, 4H), 7.40(t, J=7.8Hz, 1H), 7.24- 7.21(m,4H),7.11(t,J=6.9Hz,1H),2.89(s,3H),2.41(s,6H); 13 C NMR (75MHz, CDCl 3 )δ 190.8, 186.9, 164.6, 142.9, 142.7, 137.7, 137.5, 137.3, 129.7, 128.9, 128.8, 128.7, 127.6, 127.1, 121.3, 119.8, 116.0, 113.5, 51.7, 21.5. It can be seen that the single peaks at ...
Embodiment 3
[0042] Add bromoacetophenone (1.0mmol), ethyl azide cinnamate (0.5mmol) and pyridine (0.75mmol), DMSO (3mL) to a 25mL reaction tube, react at 100°C for 3h, track the reaction by TLC, and wait for the reaction After the end, extract with dichloromethane, anhydrous Na 2 SO 4 After drying and column chromatography (developer petroleum ether / ethyl acetate v / v=3:1), a yellow solid A3 can be obtained in a yield of 78%. The reaction principle is as follows.
[0043]
[0044] The results of the H NMR and C NMR spectra of compound A3 are as follows: Ethyl1,3-dibenzoylindolizine-2-carboxylate (A3), 1 H NMR (300MHz, CDCl 3 )δ9.54 (d, J = 6.3Hz, 1H), 8.16 (d, J = 8.7Hz, 1H), 7.69 (d, J = 8.4Hz, 3H), 7.48-7.26 (m, 8H), 7.12- 7.10(m,1H),3.20-3.13(m,2H),0.73-0.64(m,3H); 13 C NMR (75MHz, CDCl 3 )δ 191.1, 187.1, 164.1, 151.0, 140.2, 140.0, 138.0, 133.6, 132.1, 132.1, 130.1, 129.1, 128.6, 128.4, 128.2, 128.2, 127.6, 127.4, 119.8, 114.2, 13.1. It can be seen that the multiplets at 0.73-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com