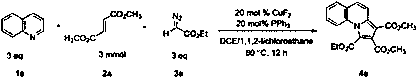Indolizine derivative and preparation method thereof
A technology of indolizine derivatives and derivatives, applied in the field of indolizine derivatives and their preparation, can solve the problems of limited application and long time, and achieve the effects of high raw material utilization, low cost, and low dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
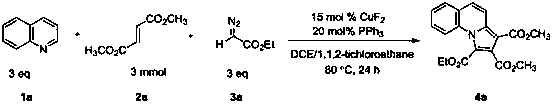
[0028] The reaction flask was filled with CuF 2 (0.6 mmol, 60 mg), PPh 3 (0.6 mmol, 157 mg), compound 1a (9 mmol, 1032 mg), compound 2a (3 mmol, 432 mg), compound 3a (9 mmol, 1026 mg), 1,2-dichloroethane (5.0 mL), 1 , 1,2-Trichloroethane (5.0 mL). Then the system was heated at 80°C in air for about 24 hours, washed with 1mol / L hydrochloric acid solution, extracted with dichloromethane (40 mL × 3), adsorbed on silica gel, and the product 4a was obtained by simple column chromatography , the yield is 87%, without PPh 3 The time yield is 75%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0029] 1 H NMR (400 MHz, CDCl3) δ 8.20 (d, J = 9.4 Hz, 1H), 8.09 (d, J = 8.7Hz, 1H), 7.75 (dd, J = 7.8, 1.3 Hz, 1H), 7.62 – 7.53 (m, 2H), 7.51 – 7.43 (m,1H), 4.43 (q, J = 7.1 Hz, 2H), 3.99 (s, 3H), 3.90 (s, 3H), 1.40 (t, J = 7.1Hz, 3H); 13 C NMR (101...
Embodiment 2
[0031]
[0032] The reaction flask was filled with CuI (0.6 mmol, 114 mg), PPh 3 (0.6 mmol, 157 mg), compound 1a (9 mmol, 1032 mg), compound 2a (3 mmol, 432 mg), compound 3a (9 mmol, 1026 mg), 1,2-dichloroethane (5.0 mL), 1 , 1,2-Trichloroethane (5.0 mL). Then the system was heated at 80°C in air for about 24 hours, washed with 1mol / L hydrochloric acid solution, extracted with dichloromethane (40 mL × 3), adsorbed on silica gel, and the product 4a was obtained by simple column chromatography , the yield is 67%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0033] 1 H NMR (400 MHz, CDCl3) δ 8.20 (d, J = 9.4 Hz, 1H), 8.09 (d, J = 8.7Hz, 1H), 7.75 (dd, J = 7.8, 1.3 Hz, 1H), 7.62 – 7.53 (m, 2H), 7.51 – 7.43 (m,1H), 4.43 (q, J = 7.1 Hz, 2H), 3.99 (s, 3H), 3.90 (s, 3H), 1.40 (t, J = 7.1Hz, 3H); 13 C NMR (101 MHz, CDCL3) Δ 165.7, 162.9, 160.3, 137....
Embodiment 3
[0035]
[0036] The reaction flask was filled with CuCl in sequence 2 (0.6 mmol, 80 mg), PPh 3 (0.6 mmol, 157 mg), compound 1a (9 mmol, 1032 mg), compound 2a (3 mmol, 432 mg), compound 3a (9 mmol, 1032 mg), 1,2-dichloroethane (5.0 mL), 1 , 1,2-Trichloroethane (5.0 mL). Then the system was heated at 80°C in air for about 24 hours, washed with 1mol / L hydrochloric acid solution, extracted with dichloromethane (40 mL × 3), adsorbed on silica gel, and the product 4a was obtained by simple column chromatography , the yield is 65%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0037] 1 H NMR (400 MHz, CDCl3) δ 8.20 (d, J = 9.4 Hz, 1H), 8.09 (d, J = 8.7Hz, 1H), 7.75 (dd, J = 7.8, 1.3 Hz, 1H), 7.62 – 7.53 (m, 2H), 7.51 – 7.43 (m,1H), 4.43 (q, J = 7.1 Hz, 2H), 3.99 (s, 3H), 3.90 (s, 3H), 1.40 (t, J = 7.1Hz, 3H); 13 C NMR (101 MHz, CDCL3) Δ 165.7, 162...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com