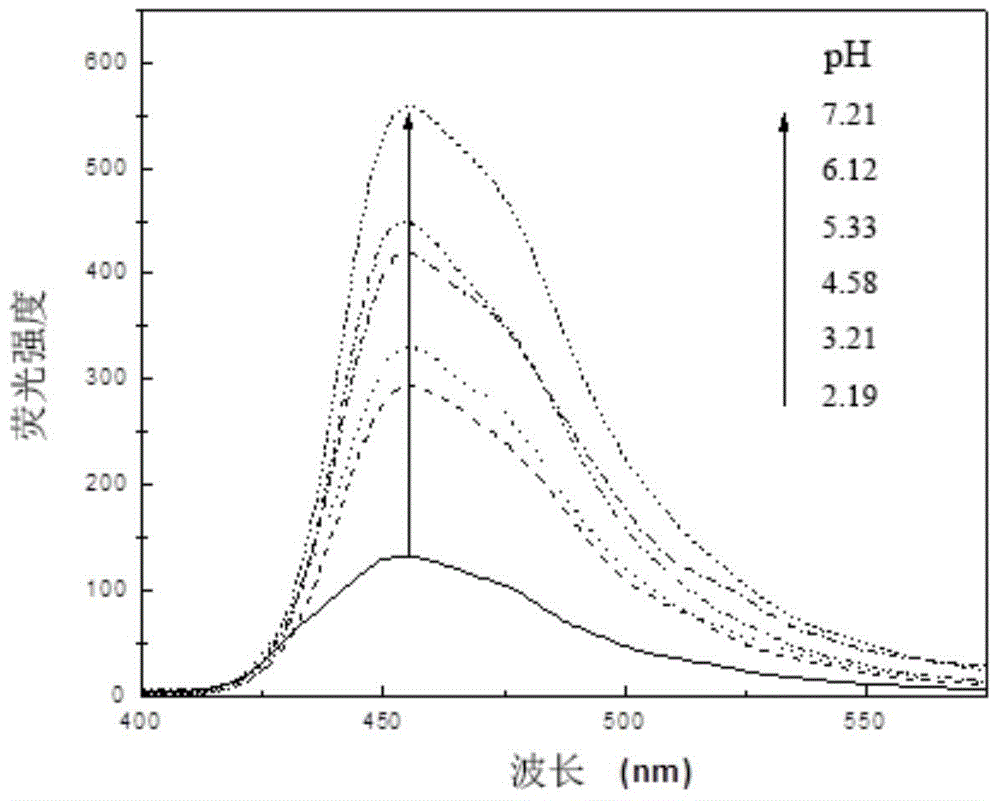
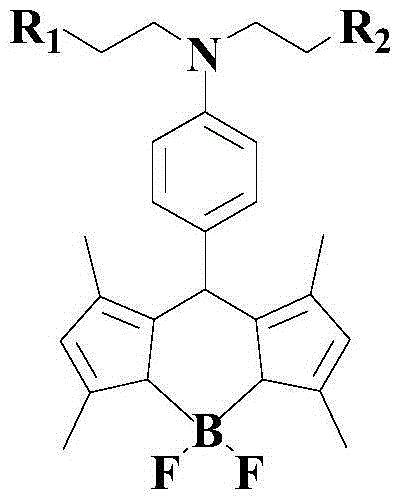
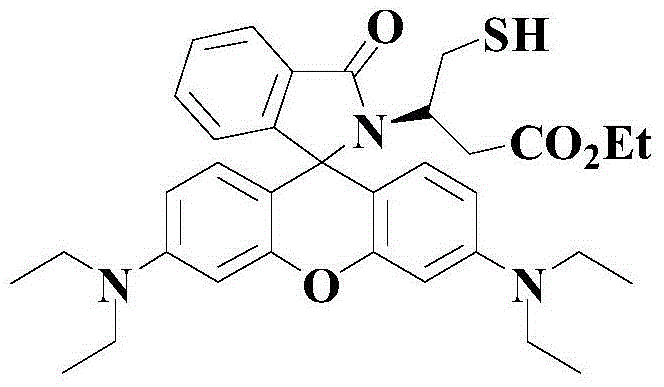
Novel pH-responsive fluorescent molecular probe and preparation method thereof
A fluorescent molecular probe and name technology, applied in the field of organic analysis and photochemistry, to overcome the influence of acid-base environment, broad industrial application prospects and scientific research value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 1mmol of formula (II) compound, 3mmol of KOH, 20mL of ethanol and 2mL of water into the reaction flask, stir, and heat to reflux until clear; then add 4.9mmol of CS 2 , continue heating to reflux, TLC tracking. Cool after reacting for 10 hours, pour into 200mL water, adjust the pH to 5-6 with 10% hydrochloric acid with a mass percent concentration, separate out a large amount of solids, filter with suction, and recrystallize the filter cake with ethyl acetate to obtain the compound of formula (I): Yellow solid, yield 81.3%.
Embodiment 2
[0037] Add 1mmol of formula (II) compound, 2mmol of KOH, 15mL of ethanol and 1mL of water into the reaction flask, stir, and heat to reflux until clear; then add 4mmol of CS 2 , continue heating to reflux, TLC tracking. Cool after reacting for 12 hours, pour into 200mL water, adjust the pH to 5-6 with 10% hydrochloric acid with a mass percent concentration, separate out a large amount of solids, filter with suction, and recrystallize the filter cake with ethyl acetate to obtain the compound of formula (I): Yellow solid, yield 78.6%.
Embodiment 3
[0039] Add 1mmol of formula (II) compound, 4mmol of KOH, 25mL of ethanol and 2.5mL of water into the reaction flask, stir, and heat to reflux until clear; then add 7mmol of CS 2 , continue heating to reflux, TLC tracking. Cool after reacting for 10 hours, pour into 200mL water, adjust the pH to 5-6 with 10% hydrochloric acid with a mass percent concentration, separate out a large amount of solids, filter with suction, and recrystallize the filter cake with ethyl acetate to obtain the compound of formula (I): Yellow solid, yield 77.4%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com