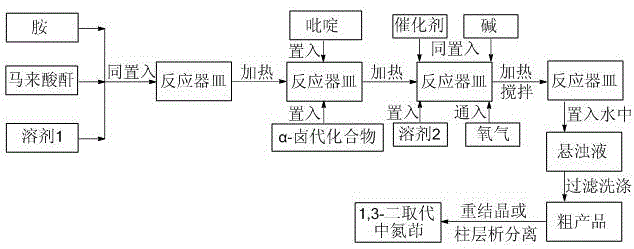
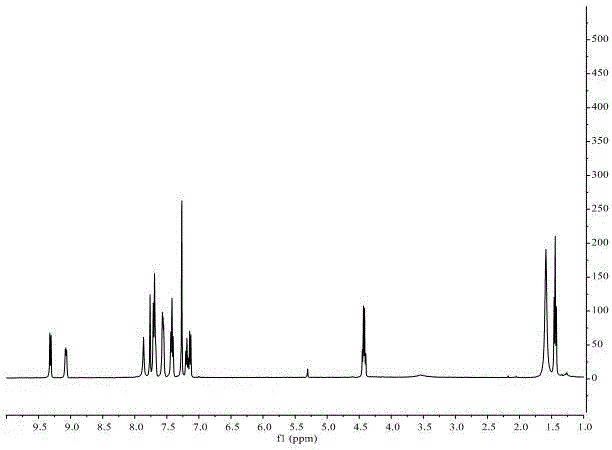
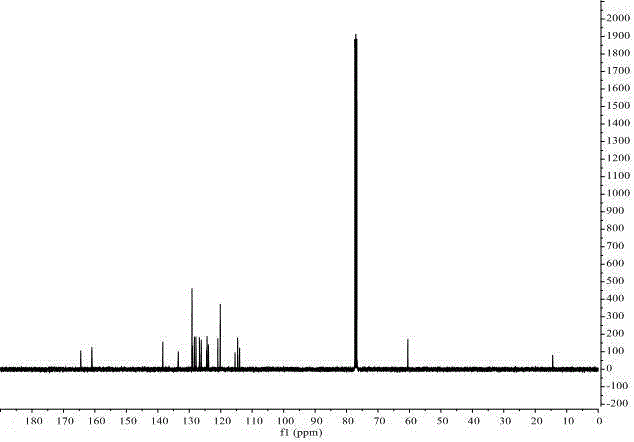
Method for preparing 1,3-disubstituted indolizine derivative
An indolizine derivative and disubstituted technology, applied in the field of organic synthesis, can solve the problems of increased synthesis cost and environmental protection cost, environmental hazards of transition metal salts, long production cycle, etc., to achieve no risk of environmental pollution, abundant sources, and improved efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] as attached figure 1 According to the technical process, 18.6 mg of aniline (equivalent to 0.20 mmol), 29.4 mg of maleic anhydride (equivalent to 0.30 mmol) and 0.20 ml of ethyl acetate were added to the container, and under stirring, the reaction was heated at 60 degrees Celsius for 3 hours. A sample spot plate showed complete reaction of the aniline. Continue to add 51.7 mg of isoquinoline (equivalent to 0.40 mmol) and 66.8 mg of ethyl α-bromoacetate (equivalent to 0.40 mmol) to the mixture obtained above, continue to heat the reaction at 60 degrees Celsius for 4 hours, take a sample point plate, and show The raw material reacted completely. 8.6 mg of cuprous bromide (corresponding to 0.06 mmol), 55.2 mg of potassium carbonate (corresponding to 0.40 mmol) and 1.8 ml of dimethyl sulfoxide were further added to the mixture obtained above, and oxygen was introduced. The mixture was heated and stirred at 100° C. for 12 hours under 1 atmospheric pressure of oxygen, and a...
Embodiment 2
[0034] as attached figure 1 The technological process of getting 4-methoxyaniline is 24.6 mg (equivalent to 0.20 mmol), maleic anhydride 19.6 mg (equivalent to 0.20 mmol) and 0.10 ml of ethyl acetate, heated at 50 degrees Celsius for 7 hours, and sampling Spot the plate, showing that the raw material has reacted completely. Continue to add 27.9 mg of 4-picoline (equivalent to 0.30 mmol) and 36.8 mg of ethyl α-chloroacetate (equivalent to 0.30 mmol) to the above-mentioned mixture, continue heating at 80 degrees Celsius for 4 hours, and take a sample point plate, It shows that the reaction of raw materials is complete. Continue to add 5.4 mg of copper acetate (equivalent to 0.03 mmol), 45.0 mg of potassium bicarbonate (equivalent to 0.45 mmol) and N , N- Dimethylformamide 2.4 ml, bubbled with oxygen. The mixture was heated and stirred at 80° C. for 16 hours under 1 atmospheric pressure of oxygen, and a plate was taken to show that the reaction of the raw materials was comple...
Embodiment 3
[0036] as attached figure 1 The technological process of getting p-chloroaniline is 25.5 mg (equivalent to 0.20 mmol), maleic anhydride 29.4 mg (equivalent to 0.30 mmol) and 0.25 milliliters of ethyl acetate, heated at 60 degrees Celsius for 5 hours, sampling point plate, It shows that the reaction of raw materials is complete. Continue to add 77.6 mg of 4-phenylpyridine (equivalent to 0.50 mmol) and 139.0 mg of α,4-dibromoacetophenone (equivalent to 0.50 mmol) to the mixture obtained above, continue heating at 70 degrees Celsius for 5 hours, and take a sample Spot the plate, showing that the raw material has reacted completely. Continue to add 4.5 mg of copper bromide (equivalent to 0.02 mmol) in the mixture obtained above, 162.9 mg of cesium carbonate (equivalent to 0.50 mmol) and N , N - Dimethylacetamide 2.0 ml, bubbled with oxygen. The mixture was heated and stirred at 100° C. for 8 hours under 1 atmospheric pressure of oxygen, and a sample was taken, which showed tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com