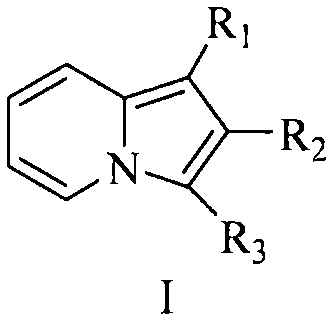
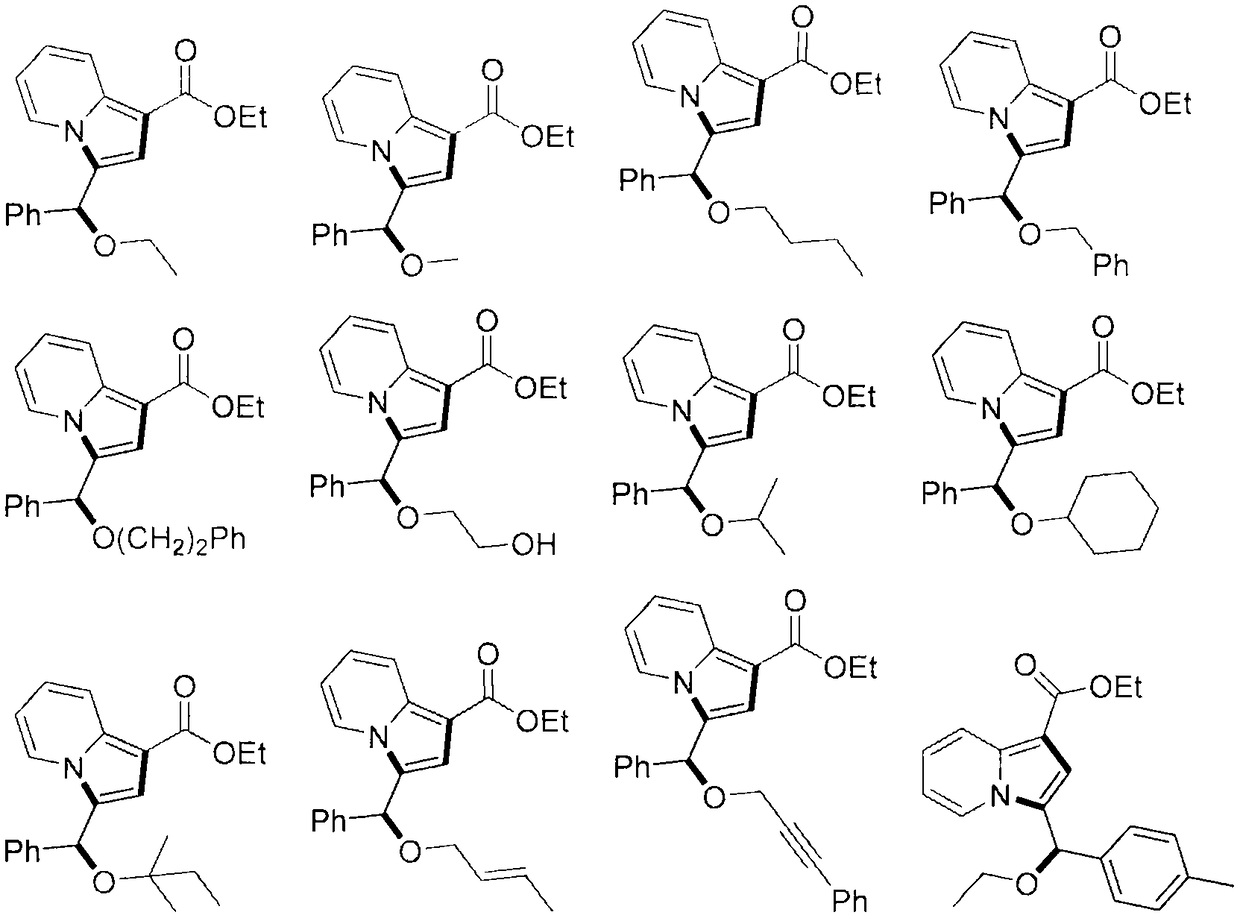
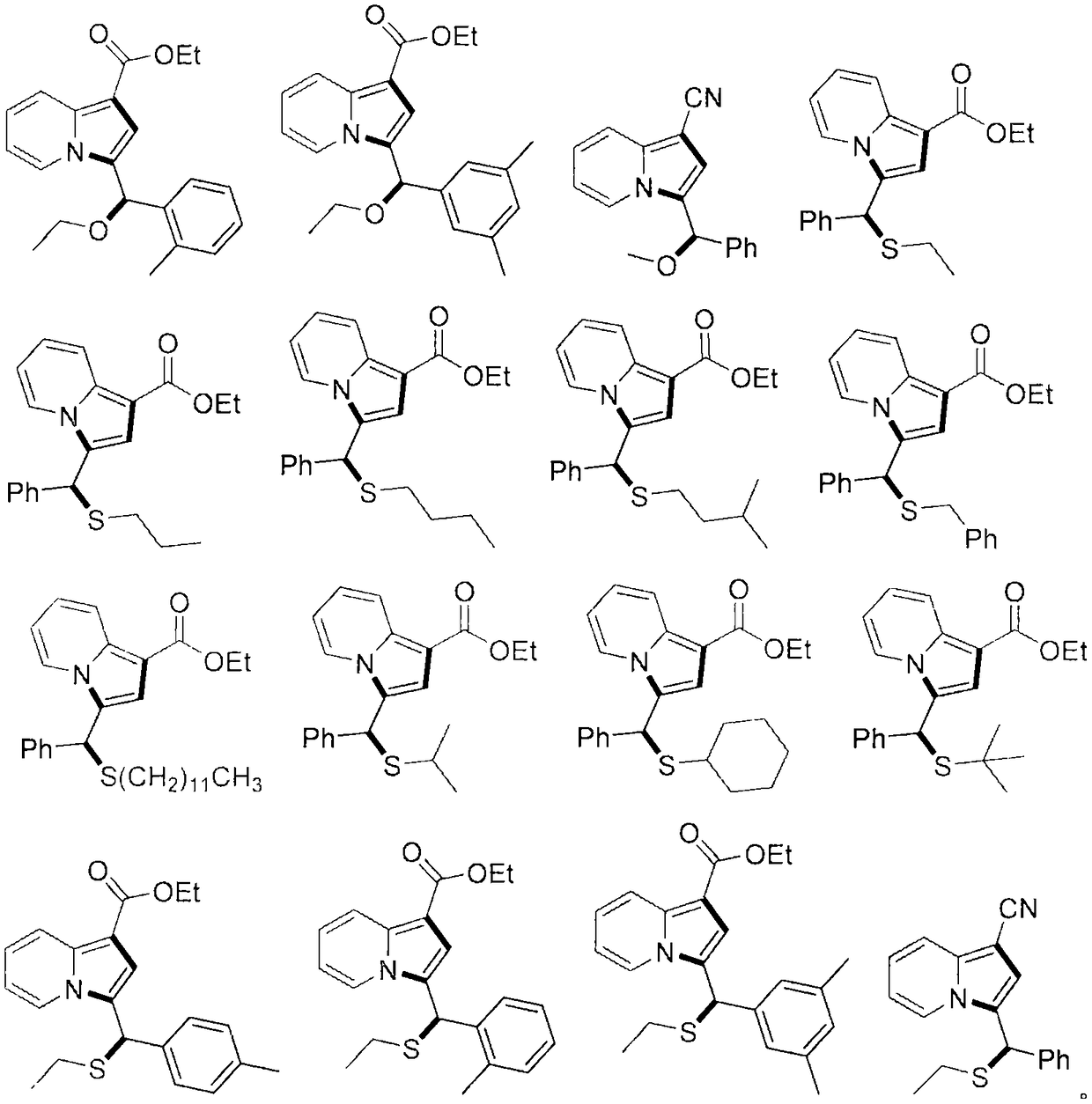
Indolizine compound with antitumor activity and derivative of indolizine compound
A kind of technology of compound and solvate, applied in the field of indolizine compounds, can solve the problems such as indolizine derivatives that have not been reported in domestic and foreign literatures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1: Ethyl 3-(ethoxy(phenyl)methyl)indazine-1-carboxylate (yield 80%)
[0069]
[0070] Add ethyl 2-pyridinecarboxylate (0.30mmol), phenylpropynaldehyde (0.30mmol), EtOH (3.0mmol), PivOH (0.20eq.), 150mg 5A molecular sieves successively in a 25ml schlenk tube, and then pass into Ar, Reaction at room temperature for 2h. After the reaction was detected by TLC, it was extracted with ethyl carboxylate (10 mL×3), the organic layers were combined and washed with saturated brine, and then washed with anhydrous MgSO 4 to dry. The obtained organic phase was distilled off under reduced pressure to remove most of the solvent, and the crude product was separated and purified by column chromatography to obtain a pure sample. The structure of the product 1 H NMR, 13 C NMR, GC-MS, HRMS and IR for analysis and confirmation.
[0071] 70.7 mg. 1 H NMR (400MHz, CDCl 3 )δ8.21(d, J=9.2Hz, 1H), 8.08(d, J=7.2Hz, 1H), 7.42-7.39(m, 5H), 7.09-7.05(m, 1H), 6.97(s, 1H ), 6.69-6.66...
Embodiment 2
[0073] Example 2: Ethyl 3-(methoxy(phenyl)methyl)indazine-1-carboxylate (yield 79%)
[0074]
[0075] 64.9 mg. 1 H NMR (400MHz, CDCl 3 )δ8.20(d, J=9.2Hz, 1H), 8.03(d, J=7.2Hz, 1H), 7.40-7.30(m, 5H), 7.09-7.05(m, 1H), 6.99(s, 1H ), 6.69-6.65(m, 1H), 5.58(s, 1H), 4.33(q, J=7.2Hz, 2H), 3.38(s, 3H), 1.37(t, J=7.2Hz, 3H). 13 C NMR (100MHz, CDCl 3 )δ165.0, 138.1, 137.1, 128.5, 128.0, 126.8, 125.0, 123.8, 122.6, 119.8, 117.8, 112.3, 102.9, 78.3, 59.5, 56.5, 14.6.
Embodiment 3
[0076] Example 3: Ethyl 3-(butoxy(phenyl)methyl)indazine-1-carboxylate (yield 77%)
[0077]
[0078] 74.8mg. 1 H NMR (400MHz, CDCl 3 )δ8.21(d, J=9.2Hz, 1H), 8.07(d, J=7.2Hz, 1H), 7.42-7.30(m, 5H), 7.09-7.05(m, 1H), 6.99(s, 1H ), 6.68-6.65(m, 1H), 5.68(s, 1H), 4.33(q, J=7.2Hz, 2H), 3.54-3.43(m, 2H), 1.65-1.58(m, 2H), 1.43- 1.36(m, 5H), 0.88(t, J=7.2Hz, 3H). 13 C NMR (100MHz, CDCl 3 )δ165.0, 138.7, 137.0, 128.5, 127.9, 126.8, 125.1, 124.2, 122.5, 119.8, 117.6, 112.1, 102.8, 76.7, 68.7, 59.4, 31.8, 19.4, 14.6, 13.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com