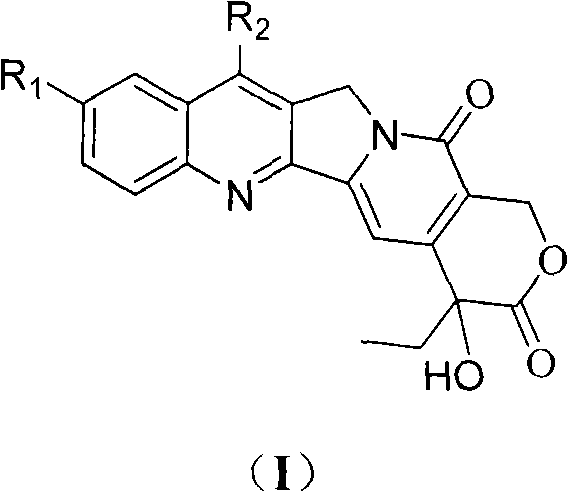
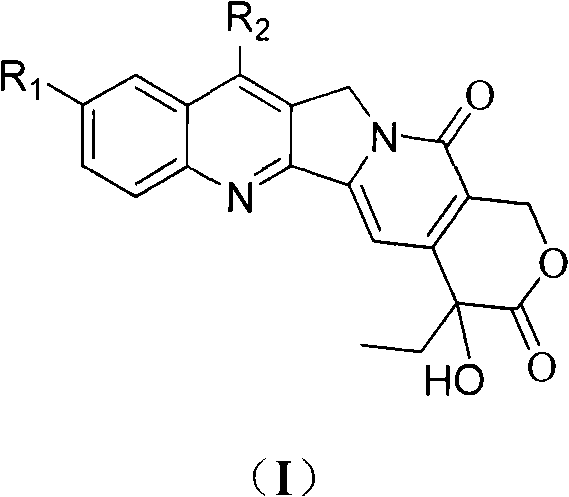
Camptothecin and method for preparing analogues thereof
A technology of analogs and camptothecin, which is applied in the field of preparation of -camptothecin and its analogs, can solve the problems of harsh reaction conditions, poor product purity, and serious waste pollution, and achieve mild reaction conditions, good product quality, The effect of less pollution of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
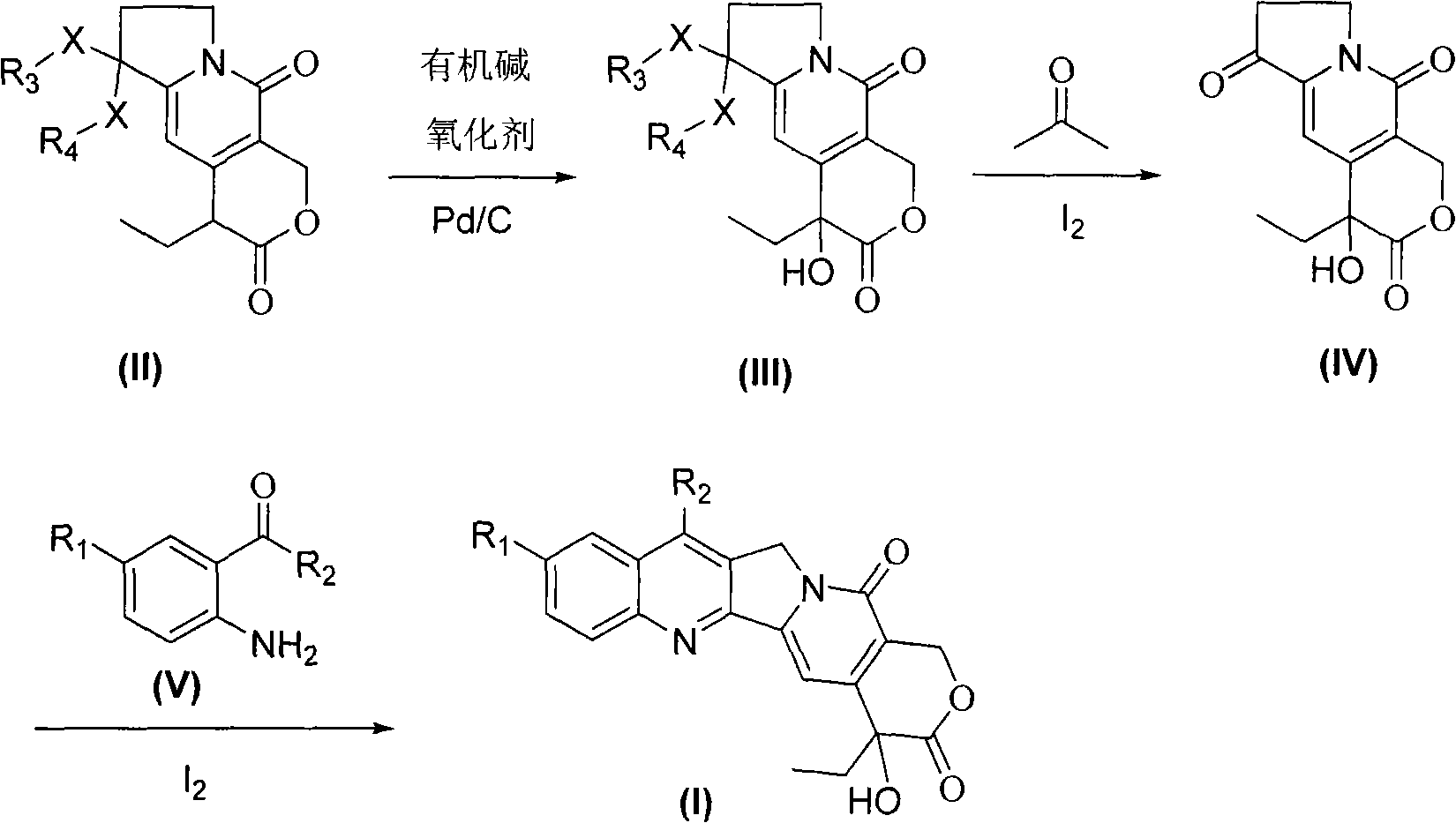
[0025] Nitrogen in 3,10-dioxo-4-ethyl-6,6-disubstituted alkoxy(thio)yl-1,4,7,8-tetrahydro-4-hydroxypyran (3,4-f) Preparation of indene (III):
[0026] Compound 3,10-dioxo-4-ethyl-6,6-ethylenedioxy-1,4,7,8-tetrahydropyran (3,4-f) indolizine (II) ( 2.91g, 10mmol), 10% palladium / carbon (0.29g), triethylamine (0.10g, 1mmol) and methanol (30mL) were placed in a reaction flask, and oxygen was introduced at 20°C under normal pressure, and the reaction was stirred for 1 hour. After completion of the reaction, filter and spin the filtrate to dryness under reduced pressure, add dichloromethane (100 mL), and wash the organic layer with water (50 mL×2) and saturated brine (50 mL), respectively. Dry over anhydrous sodium sulfate, recover dichloromethane, and obtain off-white compound 3,10-dioxo-4-ethyl-6,6-ethylenedioxy-1,4,7,8-tetrahydro-4- Hydroxypyran (3,4-f) indolizine (III) 2.7g, yield 88%, mp 178.5-180°C, purity ≥ 98%.
Embodiment 2
[0028] Compound 3,10-dioxo-4-ethyl-6,6-ethylenedioxy-1,4,7,8-tetrahydropyran (3,4-f) indolizine (II) ( 0.29g, 1mmol), the 10% palladium / carbon (0.029g) recovered in Example 1, triethylamine (10.1mg, 0.1mmol) and methanol (3mL) were placed in a reaction flask, and passed through at 25°C under normal pressure Oxygen, stirred for 2 hours. After the reaction was completed, it was filtered, and the filtrate was spin-dried under reduced pressure, dichloromethane (50 mL) was added, and the organic layer was washed with water (20 mL×2) and saturated brine (20 mL), respectively. Dry over anhydrous sodium sulfate, recover dichloromethane, and obtain off-white compound 3,10-dioxo-4-ethyl-6,6-ethylenedioxy-1,4,7,8-tetrahydro-4- Hydroxypyran (3,4-f) indolizine (III) 0.26g, yield 85%, mp 177.3-180.5°C, purity ≥ 96%.
Embodiment 3
[0030]Compound 3,10-dioxo-4-ethyl-6,6-(2′,2′-dimethyl-1′,4′propylenedioxy)-1,4,7,8- Tetrahydropyran (3,4-f) indolizine (II) (3.33g, 10mmol), 10% palladium / carbon (0.33g), triethylamine (0.10g, 1mmol) and ethanol (30mL) were reacted In the bottle, at 25°C, oxygen was introduced under normal pressure, and the reaction was stirred for 2 hours. After completion of the reaction, filter and spin the filtrate to dryness under reduced pressure, add dichloromethane (100 mL), and wash the organic layer with water (50 mL×2) and saturated brine (50 mL), respectively. Dry over anhydrous sodium sulfate, recover dichloromethane, and obtain off-white compound 3,10-dioxo-4-ethyl-6,6-(2',2'-dimethyl-1',4'-propylene Dioxy)-1,4,7,8-tetrahydro-4-hydroxypyran (3,4-f) indolizine (III) 3.12g, yield 89%, mp 188~189.7℃, purity ≥ 98%.
[0031] Example 3
[0032] Preparation of 3,6,10-trioxy-4-ethyl-1,4,7,8-tetrahydro-4-hydroxypyran (3,4-f) indolizine (IV):
[0033] The compound 3,10-dioxo-4-ethyl-6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com