Simple preparation method of 3-aryl indolizine derivative
A technology of indolizine derivatives and aryl groups, which is applied in the field of organic synthetic chemistry to achieve the effects of shortening the process flow, easy availability and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
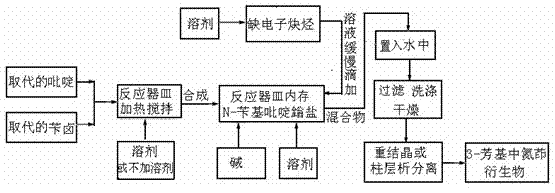
[0031] as attached figure 1 According to the technical process, 0.71 g of pyridine (equivalent to 9.0 mmol) and 1.54 g of benzyl bromide (equivalent to 9.0 mmol) were put into the reaction vessel, and 2.0 ml of N,N-dimethylformamide was added. Heat and stir at 40°C for 1.0 hour, add 1.34 g of potassium tert-butoxide (equivalent to 12.0 mmol) and 18 ml of N,N-dimethylformamide into the reaction vessel, and add 0.25 g of methyl propiolate (equivalent to 3.0 mmol) was dissolved in 10 ml of N,N-dimethylformamide, slowly added dropwise to the reaction vessel at 80 degrees, heated and stirred for 4.0 hours to complete the reaction, and the reacted mixture was poured into water, After filtering and washing, 0.38 g of the target product of this example was isolated (51% yield).
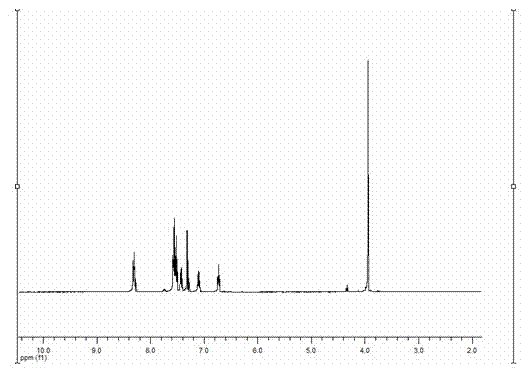
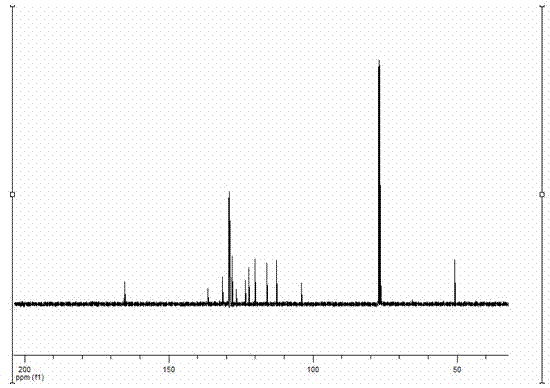
[0032] The target product in Example 1 was analyzed by a nuclear magnetic resonance spectrometer (model: AVANCE 400MHz, manufacturer: Bruker, Switzerland) to obtain figure 2 The H NMR spectra shown and ...
Embodiment 2
[0034] as attached figure 1 According to the technological process, 1.16 grams of quinoline (equivalent to 9.0 mmol) and 1.17 grams of p-methylbenzyl chloride (equivalent to 9.0 mmol) were put into the reaction vessel, and 5.0 ml of 1,4-diox Hexacyclic, heated and stirred at 40 degrees for 1.0 hours, added 2.87 g of potassium phosphate (equivalent to 13.5 mmol) and 25 ml of 1,4-dioxane to the reaction vessel, and methyl phenylpropiolate 0.72 g (equivalent to 4.5 mmol) was dissolved in 10 ml of 1,4-dioxane, slowly added dropwise to the reaction vessel at 80 degrees, heated and stirred for 4.0 hours, the reaction was completed, and the reacted mixture was poured into water, filtered, washed, and isolated to obtain 0.78 g of the target product of the example (44% yield).
Embodiment 3
[0036] as attached figure 1 According to the technological process, 1.16 grams of isoquinoline (equivalent to 9.0 mmol) and 2.25 grams of o-bromobenzyl bromide (equivalent to 9.0 mmol) were put into the reaction vessel, and 10 ml of acetonitrile was added, and the Heat and stir under temperature conditions, heat and stir for 1.0 hour, add 5.86 g of cesium carbonate (equivalent to 18 mmol) and 30 ml of acetonitrile into the reaction vessel, dissolve 1.77 g of butynoate methyl ester (equivalent to 18 mmol) in 20 ml Acetonitrile was slowly added dropwise to the reaction vessel at 80 degrees, and the heating and stirring time was 4.0 hours. The reaction was completed, and the reacted mixture was poured into water, filtered, washed, and separated to obtain 2.02 grams of the target product of this embodiment (yield: 57 %).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com