Acidification corrosion inhibitor based on interpolymer indolizine derivative as well as preparation method and application thereof
A technology of acidification corrosion inhibitor and indolezine, which is applied in the field of acidification corrosion inhibitor and its preparation, can solve problems such as application limitations, and achieve the effects of small impact, environmental friendliness and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
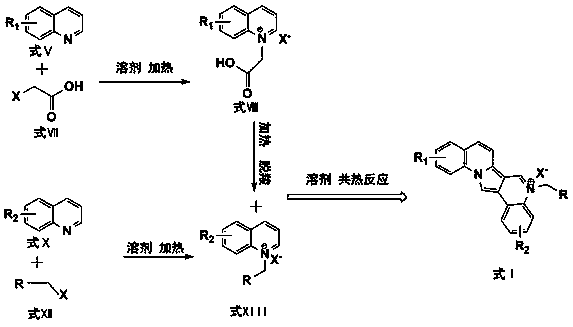
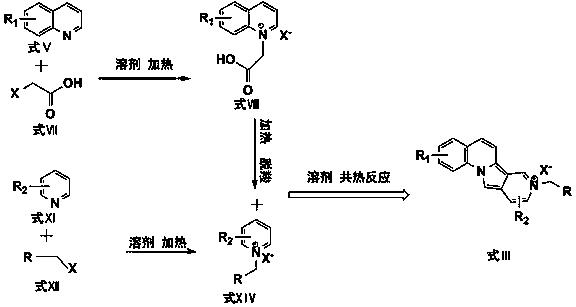
[0041] a. Take pyridine and bromoacetic acid with a molar ratio of 1:1, use the same molar amount of water as a solvent, heat and reflux at 50°C and stir for 12 hours to obtain a light yellow liquid, which is a brominated carboxymethylpyridine quaternary Ammonium salt solution. The water in the above-mentioned quaternary ammonium salt solution was heated and evaporated to obtain light yellow-white carboxymethylpyridinium bromide quaternary ammonium salt solid.
[0042] b. Take pyridine and benzyl bromide with a molar ratio of 1:1 (the molar weight is the same as the first step in Example 1), and use equimolar water as a solvent to reflux and stir at 90-100 ° C for 8 hours to carry out quarterly The corresponding quaternary ammonium salt solution is obtained through the ammonization reaction, and the appearance is a dark yellow to reddish-brown transparent liquid. The solvent was evaporated under heating to finally obtain an orange-yellow benzylpyridinium bromide quaternary ammo...
Embodiment 2
[0046] a. Take quinoline and bromoacetic acid with a molar ratio of 1:1, and use the same molar amount of water as a solvent to reflux and stir at 50°C for 12 hours to obtain a light yellow liquid, which is carboxymethylquinoline bromide Quaternary ammonium salt solution. The solvent water in the above-mentioned quaternary ammonium salt solution was heated and evaporated to obtain light orange-yellow carboxymethylquinoline bromide quaternary ammonium salt solid.
[0047] b. Take quinoline and benzyl bromide with a molar ratio of 1:1 (the molar weight is the same as the first step in Example 2), and use equimolar water as a solvent to reflux and stir at 80-90 ° C for 6 hours. Quaternization reaction to obtain the corresponding quaternary ammonium salt solution, the appearance is orange-red transparent liquid. The solvent was evaporated under heating to give a light brown benzylquinoline quaternary ammonium bromide solid.
[0048] c. Mix the solids obtained in the two-step rea...
Embodiment 3
[0051] a. Take pyridine and chloroacetic acid with a molar ratio of 1:1, use the same molar amount of water as a solvent, heat and reflux at 50°C and stir for 12 hours to obtain a light yellow liquid, which is a quaternary carboxymethylpyridine chloride. Ammonium salt solution. The water in the above-mentioned quaternary ammonium salt solution was heated and evaporated to obtain light yellow-white carboxymethylpyridinium chloride quaternary ammonium salt solid.
[0052] b. Take pyridine and ethyl α-chloroacetate with a molar ratio of 1:1 (the molar weight is the same as the first step in Example 3), and use equimolar water as a solvent to reflux and stir at 90-100°C for 8 Hours of quaternization reaction to obtain the corresponding quaternary ammonium salt solution, the appearance is dark yellow to reddish-brown transparent liquid. The solvent was evaporated under heating to finally obtain an orange-yellow solid of ethyl acetate pyridinium chloride quaternary ammonium salt. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com