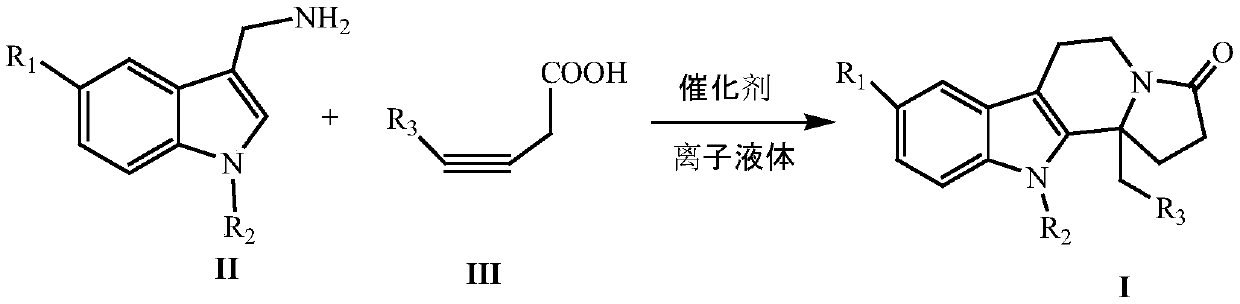
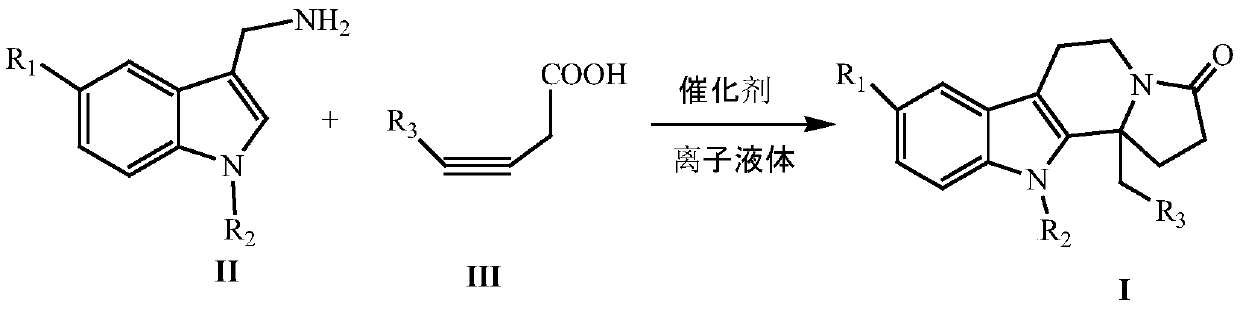
Synthesizing method of indole and indolizine ketone compounds
A technology of indolizinone and synthesis method, applied in the direction of organic chemistry and the like, can solve problems such as no literature report, and achieve the effects of rich variety, concise and efficient synthesis method, and wide adaptability of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
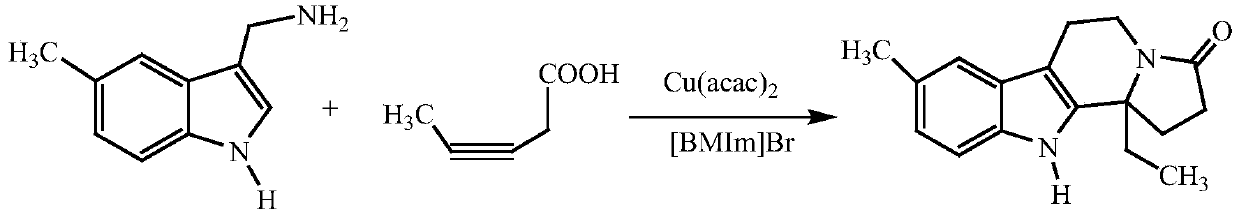
Embodiment 1
[0018] The reaction formula is:
[0019]
[0020] Add 10mmol (5-methyl-1H-indol-3-yl)methanamine, 12mmol 3-pentynoic acid and 10mL ionic liquid [BMIm]Br into the reaction flask, stir until dissolved, then add 0.5mmol catalyst Cu( acac) 2 , continue to stir for about 0.5h, then raise the temperature to 70°C, stir for another 6h, and detect the reaction progress by thin-layer chromatography. Add 20mL of ethyl acetate in three times, combine the organic layer, wash the organic layer twice with saturated brine, combine the organic layer, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure to obtain a solid, use 95% ethanol as solvent Recrystallization was carried out to obtain the target product with a yield of 92%.
Embodiment 2
[0022] The reaction formula is:
[0023]
[0024] Add 10mmol (5-chloro-1H-indol-3-yl)methanamine, 12mmol 3-butynoic acid and 10mL ionic liquid [HMIm]Cl into the reaction flask, stir until dissolved, then add 0.6mmol catalyst Vo(acac ) 2 , continue to stir for about 0.5h, then raise the temperature to 80°C, stir and react for 8h, and detect the reaction process by thin-layer chromatography. Add 20 mL of ethyl acetate in three times, combine the organic layer, wash the organic layer twice with saturated brine, combine the organic layer, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure to obtain a solid, and use 95% ethanol as the solvent for After recrystallization, the target product was obtained with a yield of 86%.
Embodiment 3
[0026] The reaction formula is:
[0027]
[0028] Add 10mmol (5-methoxy-1H-indol-3-yl)methanamine, 12mmol 4-(p-tolyl)-3-butynoic acid and 10mL ionic liquid [BMMIm]Br into the reaction flask, stir to dissolve Then add 0.8mmol catalyst Ni(acac) 2 , continue to stir for about 0.5h, then raise the temperature to 75°C, stir for another 7h, and detect the reaction process by thin-layer chromatography. Add 20mL of ethyl acetate in three times, combine the organic layer, wash the organic layer twice with saturated brine, combine the organic layer, dry over anhydrous sodium sulfate, evaporate the solvent under reduced pressure to obtain a solid, use 95% ethanol as solvent Recrystallization was carried out to obtain the target product with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com