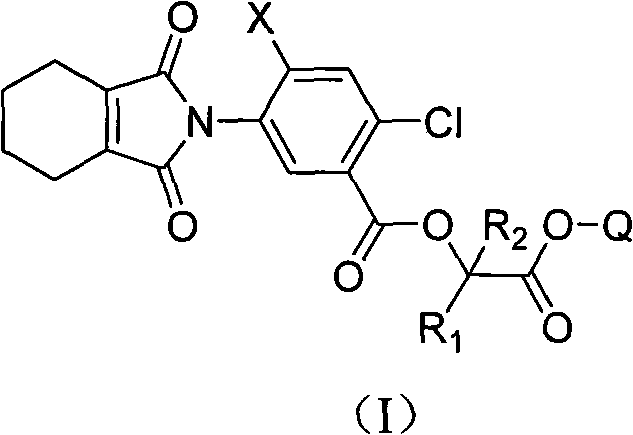
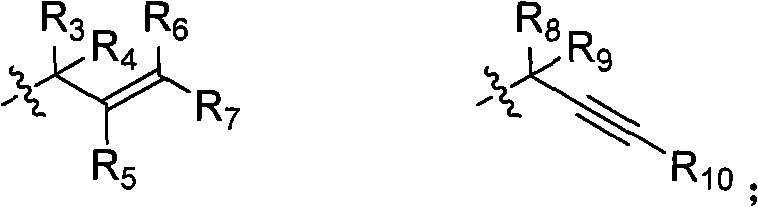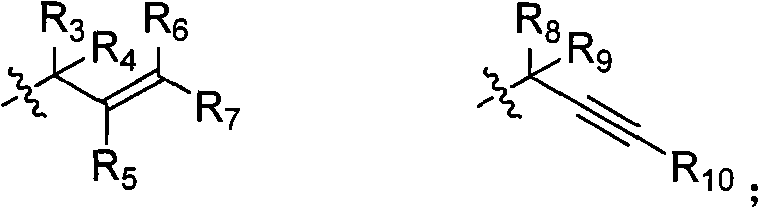Isoindole compounds and uses thereof
A compound, the technology of isoindole, which is applied in the field of isoindole compounds and their applications, can solve problems not involved in the preparation and application of isoindole compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Embodiment 1: the synthesis of compound 1
[0107]
[0108] Add 2.0 g (21 mmol) of chloroacetic acid, 20 ml N,N-dimethylformamide, 2.8 g (20 mmol) of potassium carbonate and 3.0 g (20 mmol) of propargyl bromide in sequence in a 100 ml reaction flask. mol), stirred at room temperature for 3 hours. The reaction solution was poured into 20 ml of water, extracted with 150 ml of ethyl acetate, the organic layer was washed with saturated brine, dried over anhydrous magnesium sulfate, and the organic solution was concentrated under reduced pressure to obtain 1.8 g of propargyl chloroacetate, a yellow liquid, and the yield : 69%.
[0109]
[0110] Add successively 0.36 grams of substituted benzoic acid (1 mmol, made with reference to the operating method provided by EP68822), 5 milliliters of N,N-dimethylformamide, 0.17 grams of potassium carbonate (1.2 mmol) in a 100 ml reaction flask, 0.40 g (3 mmol) of propargyl chloroacetate was stirred at room temperature for 1 hou...
Embodiment 2
[0111] Embodiment 2: the synthesis of compound 16
[0112]
[0113] Add 1.06 g (10.0 mmol) of lactic acid, 10 ml of N, N-dimethylformamide, 1.46 g (11.0 mmol) of potassium carbonate, and 0.8 g (11.0 mmol) of allyl chloride in a 100 ml reaction flask. . Stir at room temperature for 4 hours. The reaction solution was poured into 20 ml of water, extracted with 100 ml of ethyl acetate, the organic layer was washed with saturated aqueous sodium chloride, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to obtain 0.95 g of allyl lactate, a yellow oil , yield 73%.
[0114]
[0115] Add 0.62 g (2.0 mmol) of substituted benzoic acid, 10 ml of dichloromethane, 0.38 g (3.0 mmol) of oxalyl chloride and 1 drop of N, N-dimethylformamide successively in a 100 ml reaction flask, and stir at room temperature 1 hour. The reaction solution was concentrated under reduced pressure to obtain 0.67 g of substituted benzoyl chloride (hereinafter referred to as ...
Embodiment 3
[0118] Embodiment 3: the synthesis of compound 23
[0119]
[0120] Add 25 grams (0.2 moles) of S-lactic acid, 59 grams (1 mole) of propylene alcohol, and 50 milliliters of benzene in a 500 milliliter reaction flask in sequence, then add 1 milliliter of concentrated sulfuric acid, heat to reflux, and use a water separator to separate the generated water, heated to reflux for 12 hours and the reaction was basically complete. The solvent was removed under reduced pressure to obtain a black oil. Distilled under reduced pressure, collecting 50-55 ° C fraction (2mmHg). 15 g of S-allyl lactate was obtained as a colorless liquid, yield: 56%. [α] D 20 =-9.8° (c=0.2, CHCl 3 ).
[0121]
[0122] Add 0.40 g (3.0 mmol) of S-allyl lactate, 10 ml of dichloromethane and 0.32 g (3.2 mmol) of triethylamine successively in a 100 ml reaction flask, and add 0.65 g (2.0 mmol) of acid chloride dropwise A solution of dichloromethane (10 ml) was added dropwise and stirred at room tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com