Polyvalent protein complex
a protein complex and polypeptide technology, applied in the field of polypeptide complexes, can solve the problem that non-covalently associated molecules are not sufficiently stable under physiological conditions to have any practical us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
BS14HP, a Bispecific Trivalent Heterodimer
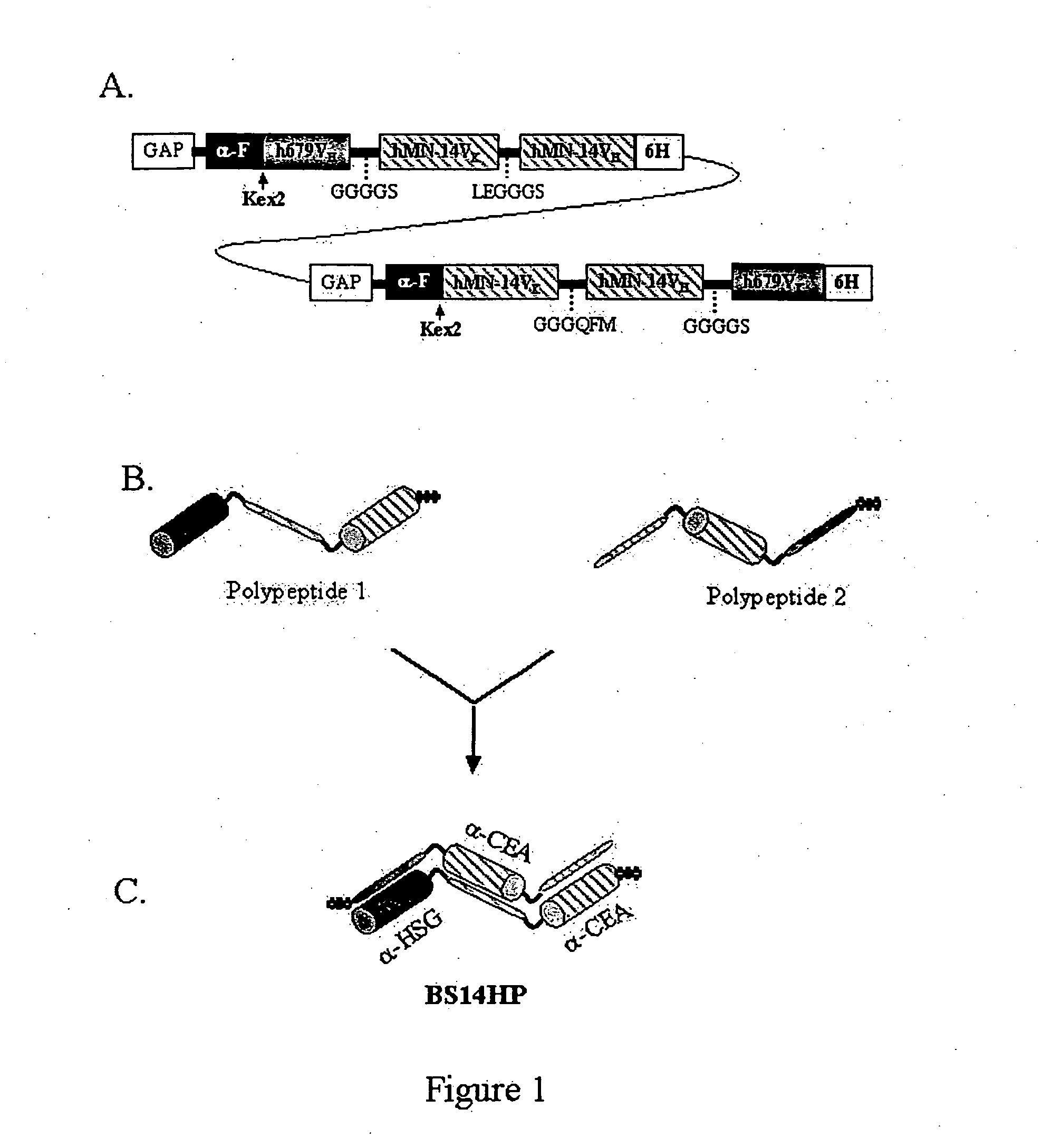
[0265] Design.
[0266] BS14HP was designed for the constitutive expression of foreign genes in Pichia pastoris using the GAP promoter system. Transfection of P. pastoris cells with a linearized DNA plasmid (BS14HP-GAP+) results in the stable and site-specific integration of the two DNA segments (FIG. 1A) into the GAP locus of the host's chromosome. These two DNA segments contain open reading frames, SEQ ID NO:1 and SEQ ID NO:2, which codes for polypeptide 1 (SEQ ID NO:9) and polypeptide 2 (SEQ ID NO:10) respectively. As each of the two DNA segments also contains nucleotide sequences for the GAP promoter, two mRNA species that encode the amino acid sequences of polypeptide 1 and polypeptide 2 are synthesized in the same host cell.
[0267] Polypeptide 1.
[0268]α-Factor-h679VH-GGGGS-hMN-14VK-LEGGGS-hMN-14VH-6His (SEQ ID NO:1)
[0269] Polypeptide 2.
[0270]α-Factor-hMN-14VK-GGGQFM-hMN-14VH-GGGGS-h679VK-6His (SEQ ID NO:2)
[0271] The “α-factor,” as s...
example 2
hBS14, a Bispecific Trivalent Heterodimer Expressed in Myeloma Cells
[0300] To demonstrate that similar PPCs could be made from other types of host cell systems we developed a scheme for production of a fusion protein named hBS14 in mammalian cell culture. The hBS14 PPC produced in mammalian cell culture was designed to be structurally and functionally similar to BS14HP, which was produced in the yeast P. pastoris (example 1). A DNA plasmid vector was engineered for hBS14 expression and used to generate transgenic cell lines in SP2 / 0-Ag14 mouse myeloma cells, NS0 mouse myeloma, and YB2 / 0 rat myeloma cells. While these particular cell lines were used, it is understood that the vectors of the invention may be used in any mammalian cell lines, such as, for example, a human cell line.
[0301] Generation of an hBS14 DNA Expression Vector
[0302] The nucleic acid encoding the hBS14 polypeptides were recombinantly inserted into the mammalian expression vector pdHL2, which permits the amplifi...
example 3
Affinity Purification of hBS14
[0317] hBS14 was purified to homogeneity using a novel affinity resin that was prepared and used as described below.
[0318] Activation and Coupling of IMP291-affigel
[0319] IMP291 peptide (see structure in FIG. 18) was coupled to Affigel 102 (BIO-RAD Laboratories, Hercules Calif.) using chloroacetic anhydride (CAA). CCA (1.5 g, 8.8 mmol) was dissolved in acetonitrile and added to 30 ml of Affigel 102 slurry. The pH was adjusted to 9.0 with triethylamine and reacted for 1 hour at room temperature to allow coupling of CAA to amine groups on the Affigel. The CAA-Affigel was washed and exchanged into 0.2M NaBorate, pH 8.0. A total of 166 mg of IMP291 was dissolved in 10 ml of 0.2M NaBorate, pH 8.0 and then added to the slurry, which was then rocked overnight at room temperature to allow coupling of the peptide to the CAA-Affigel via thioether bond formation. The resin was quenched by adding cysteine in 0.2M NaBorate, pH 8.0 to a final concentration of 20 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com