Puerarin glycosylation derivative, medicine compound, preparation method and application thereof
A technology of puerarin glycosylation and derivatives, applied in the field of medicine, can solve the problems of reduced drug safety, fast elimination rate, adverse reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
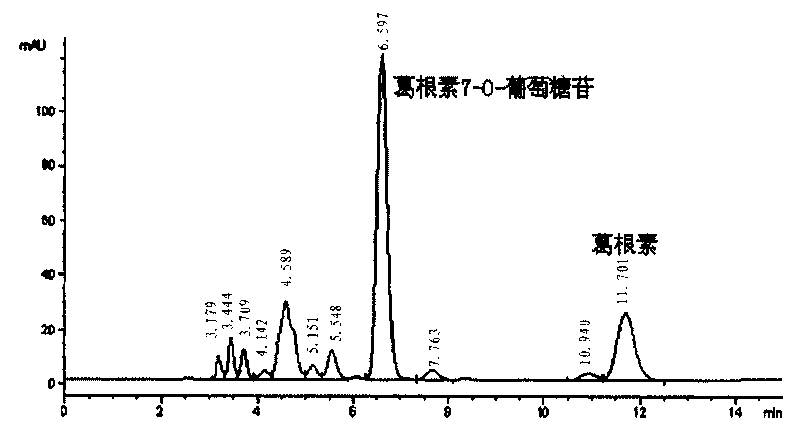
[0054] Inoculate the strain M. oxydans CGMCC 1788 in a 1000mL Erlenmeyer flask containing 300mL LB medium, and culture at 30°C with shaking at 220rpm for 24 hours; then transfer the cultivated culture solution into a 5L fermenter containing 3L LB at a speed of 500rpm , temperature 30°C, air flow 3L / min, tank pressure maintained at 0.1MPa, fermentation for 11-12h; then, centrifuge at 8000rpm for 10min to collect bacteria, wash once with 1 / 15M PBS (pH8.0), and centrifuge again to collect. The bacteria were transferred to 3L transformation solution, which contained 4mg / mL puerarin, 6% sucrose, 1 / 15M PBS, pH8.0, and transformed in a 5L reaction vessel at 500rpm, 30°C, and 3L / min ventilation After 48 hours, stop the reaction when the conversion rate of puerarin-7-O-glucoside reaches about 50%. Boil the transformation solution at 100°C for 10 minutes, cool to room temperature and centrifuge at 8000rpm for 10 minutes to remove bacteria and precipitates, take the supernatant transform...
Embodiment 2
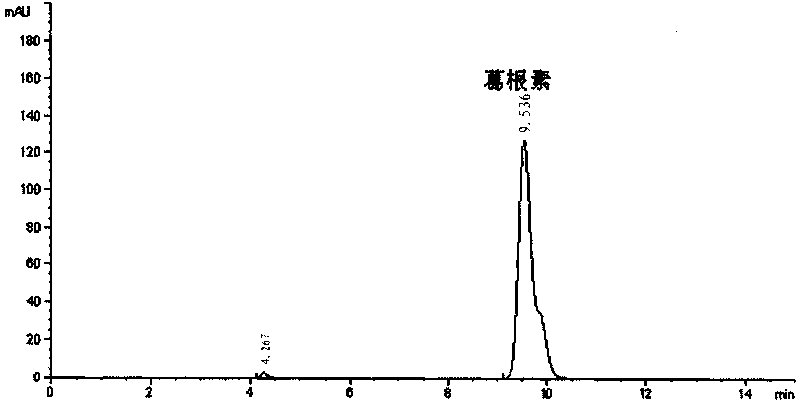
[0063] The bacterial strain M.oxydans CGMCC 1788 was inoculated in a 250mL Erlenmeyer flask containing 00mL LB medium, 30°C, 220rpm shaking culture for 12 hours, and then, 1000rpm centrifuged for 10min to collect the bacteria, and 1 / 15M phosphate buffer solution (pH8.0 ) was washed once, and after centrifugation again, the obtained bacterial cells were suspended in 100 milliliters of 1 / 15M phosphate buffered saline solution. After the cells were broken using a Franch Press high-pressure cell disruptor, the cell fragments were removed by centrifugation at 12000 rpm, and the supernatant cell extract was collected. Add 100ml of transformation liquid, the transformation liquid contains 4mg / mL of puerarin, 6% sucrose, 1 / 15MPBS, pH8.0, 30°C, 220rpm shaking for 48h, the HPLC spectrum measured after the transformation liquid is diluted 100 times is shown in figure 2 . Boil the transformation solution at 100°C for 10 minutes, cool to room temperature and centrifuge at 8000rpm for 10 m...
Embodiment 3
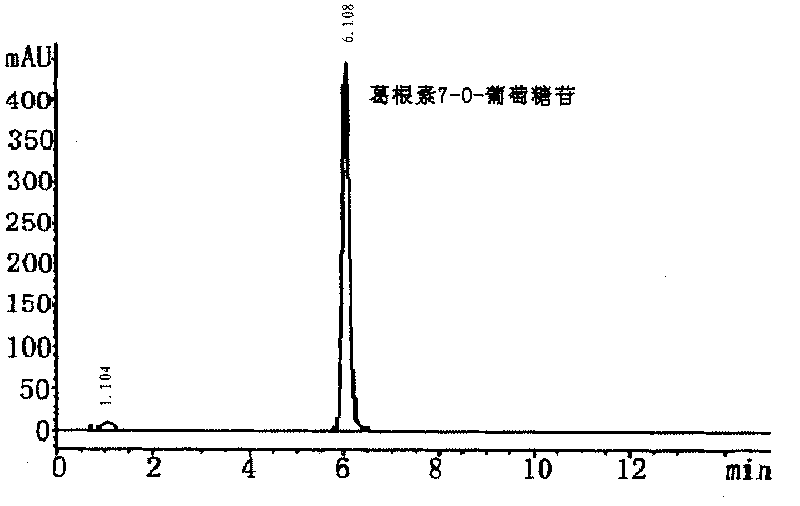
[0072] Put 150mL of LB medium into a 500mL Erlenmeyer flask, a total of 1L of medium. After high-pressure steam sterilization at 121°C, insert Microbacterium oxydans CGMCC1788 seed solution, 30°C, 220rpm shaking culture for 12 hours, stop fermentation, collect bacterial cells by centrifugation, put them into a 500mL Erlenmeyer flask, add 150mL containing 0.02% puerarin -2% maltose 1 / 15mol / L phosphate buffer solution (pH8.0), 30°C, 200rpm shaking aeration to carry out the puerarin glycosylation resting cell transformation reaction, after 48h, stop the transformation reaction, centrifuge to remove the bacteria, The supernatant was separated by C18 preparative high-performance liquid chromatography to obtain puerarin-7-O-glucoside and puerarin-7-O-isomaltoside components respectively. °C until crystals are precipitated, the crystals are collected, washed and dried to obtain a product, and the contents of puerarin-7-O-glucoside crystals and puerarin-7-O-isomaltoside crystals are b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com