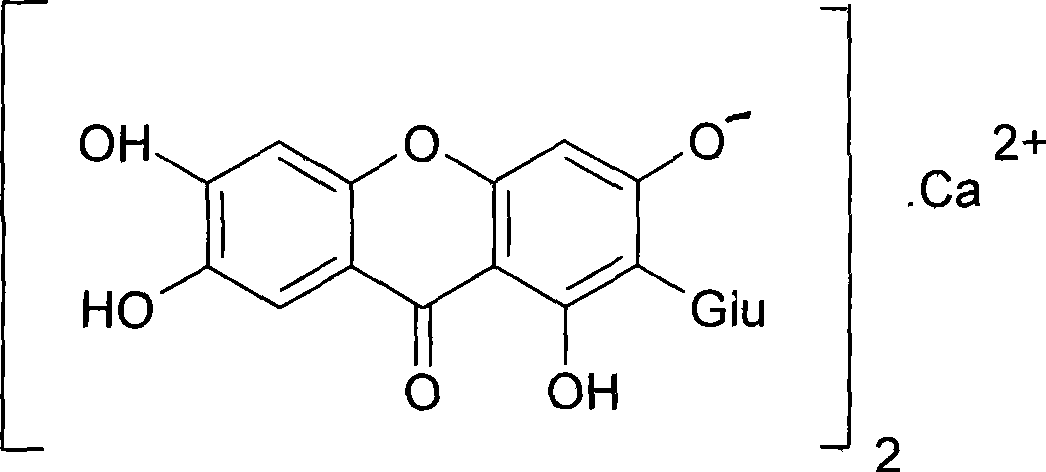
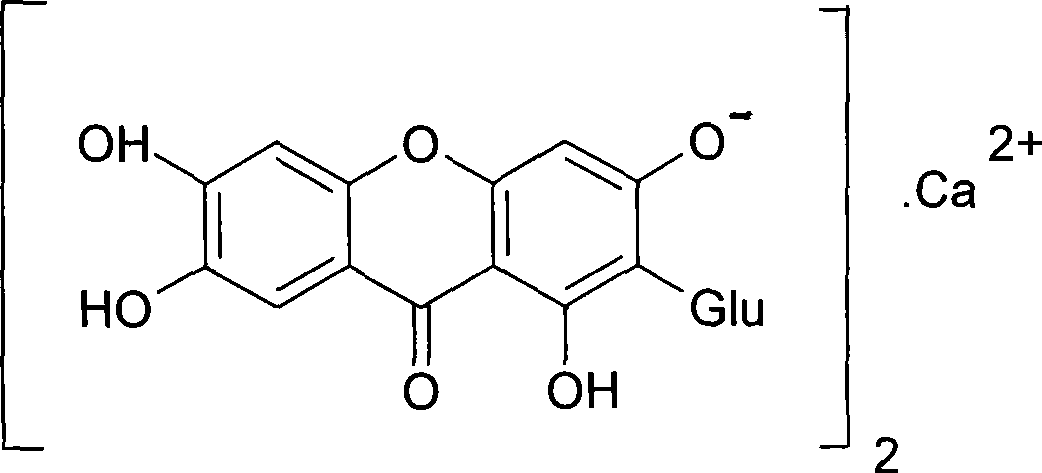
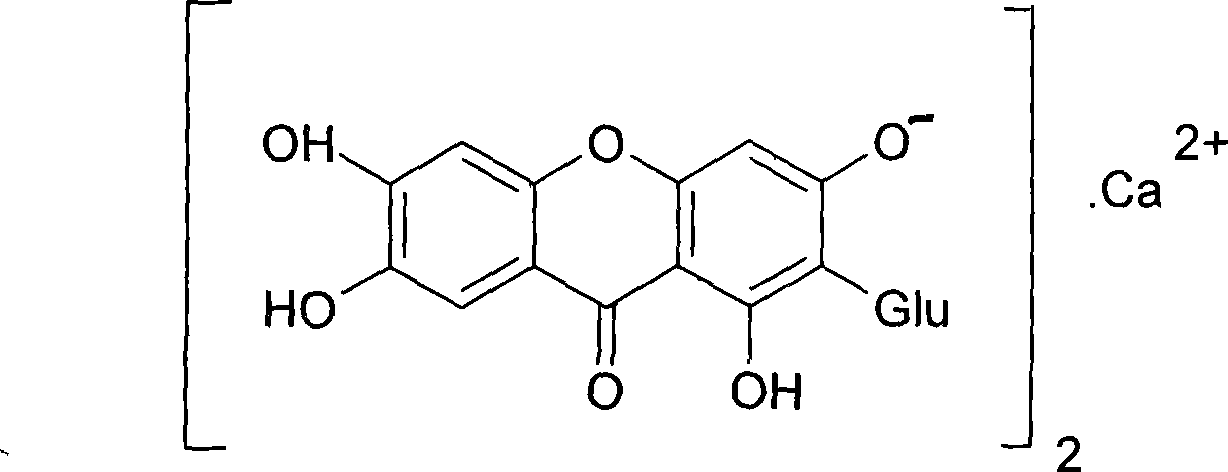
Use of mangiferin calcium salt as peroxisome proliferator-activated receptor agonist
A peroxidase and receptor agonist technology, applied in the field of mangiferin calcium salt as a peroxidase proliferator-activated receptor agonist, can solve problems such as adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation of mangiferin
[0027] 100kg Anemarrhena decoction pieces were added with 80% ethanol to reflux extraction twice, each time for 1 hour, the ethanol was recovered under reduced pressure, adsorbed by macroporous resin, washed with water, eluted with 20% ethanol, and the eluate was concentrated under reduced pressure to obtain crude neomangiferin; Then eluted with 40% ethanol, and the eluate was concentrated under reduced pressure to obtain crude mangiferin. The crude mangiferin was recrystallized and refined to obtain pure mangiferin, and the mangiferin sample was identified with the mangiferin reference substance to confirm that the obtained sample was mangiferin, and the purity was greater than 90% as determined by HPLC. Embodiment 2: the preparation of mangiferin calcium salt
Embodiment 2
[0027] 100kg Anemarrhena decoction pieces were added with 80% ethanol to reflux extraction twice, each time for 1 hour, the ethanol was recovered under reduced pressure, adsorbed by macroporous resin, washed with water, eluted with 20% ethanol, and the eluate was concentrated under reduced pressure to obtain crude neomangiferin; Then eluted with 40% ethanol, and the eluate was concentrated under reduced pressure to obtain crude mangiferin. The crude mangiferin was recrystallized and refined to obtain pure mangiferin, and the mangiferin sample was identified with the mangiferin reference substance to confirm that the obtained sample was mangiferin, and the purity was greater than 90% as determined by HPLC. Embodiment 2: the preparation of mangiferin calcium salt
[0028] Mangiferin calcium salt can be prepared according to the preparation method contained in the patent [PCT / CN2007 / 071112] applied by the inventor. Here, only one method is provided to illustrate the acquisition ...
Embodiment 3
[0044] Example 3: Preparation of mangiferin calcium salt freeze-dried powder injection
[0045] Weigh 40g of mannitol, put it in an appropriate container, add 1000ml of water for injection, add 0.2g (0.1% w / v) of charcoal for needles, heat to 80°C, stir for 30min, filter through a 0.22μm microporous membrane, and set aside the filtrate.
[0046] Weigh 10g of mangiferin calcium salt, add it into the mannitol solution, and stir to dissolve completely. Add water for injection to 2000ml, check the content and pH value, filter with a 0.22μm microporous membrane, sub-package, each tube contains G10mg, and freeze-dry. Vacuum plugging, capping, labeling, and packaging.
[0047]In order to illustrate the pharmacological activity of the calcium salt of mangiferin described in the summary of the invention, the inventors conducted experimental research in a combination of in vivo and in vitro, using representative disease models as representatives of various diseases. That is, to study ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com