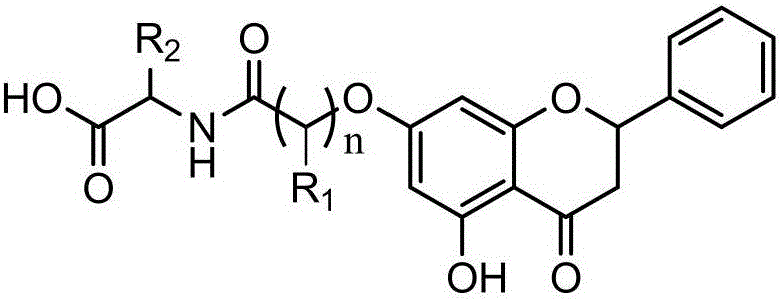
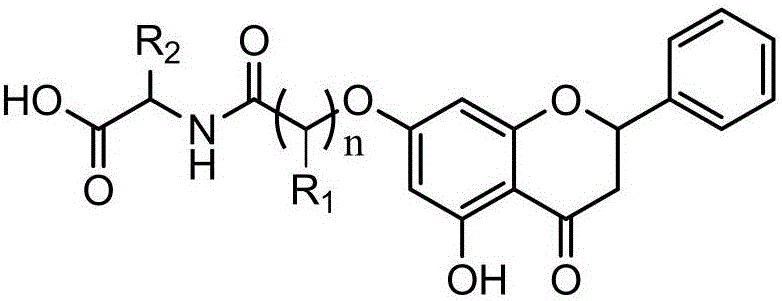
Preparation method of chrysin amino acid derivative
A technology of chrysin and its derivatives, which is applied in the field of chemistry, can solve the problems of low chrysin derivative utilization, inability to improve the solubility of chrysin, and inability to reduce chrysin, and achieve low investment, significant killing effect, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] Such as figure 1 As shown, the preparation method of the chrysin amino acid derivative provided by the embodiment of the present invention, the specific implementation steps include:
[0031] S101. Put 10mmol of chrysin and 11.5mmol of anhydrous potassium carbonate in a 250mL three-necked bottle, add 100mL of solvent acetone, heat and stir under reflux for 1h, then slowly add bromoalkyl to the reaction solution with a constant pressure dropping funnel Carboxylate and catalytic amount of KI 1mmol, continue to condense and reflux at about 60°C, and check the reaction progress by TLC. After the reaction, cool, filter, add appropriate amount of acetone to the filter residue, stir to dissolve and filter again, combine the two filtrates, evaporate the acetone under reduced pressure, and obtain a yellow crude product, which is recrystallized with acetone or separated and purified by silica gel chromatography (eluent: V 石油醚 :V 乙酸乙酯=4:1) to obtain the intermediate product (2 s...
Embodiment 1
[0036] Example 1: Preparation of ethyl 2-(5-hydroxy-2-phenyl-4H-benzopyrone-7-oxo)propionate
[0037]
[0038] Put 10mmol of chrysin and 11.5mmol of anhydrous potassium carbonate in a 250mL three-neck flask, add 100mL of solvent acetone, heat and stir to reflux for 1h, then slowly add 2-bromopropionate ethyl to the reaction solution dropwise with a constant pressure dropping funnel The ester and the catalytic amount of KI1mmol were condensed and refluxed at about 60°C, and the reaction progress was detected by TLC. After the reaction, cool, filter, add appropriate amount of acetone to the filter residue, stir to dissolve and filter again, combine the two filtrates, evaporate the acetone under reduced pressure, and obtain a yellow crude product, which is recrystallized with acetone or separated and purified by silica gel chromatography (eluent: V 石油醚 :V 乙酸乙酯 =4:1) Compound 2 was obtained with a yield of 70.5%. m.p.138.5~140.2℃.IR v max (cm -1 , KBr): 1738, 1657, 1616, 13...
Embodiment 2
[0039] Example 2: Preparation of ethyl 4-(5-hydroxy-2-phenyl-4H-benzopyrone-7-oxo)butanoate
[0040]
[0041] The preparation method was the same as in Example 1, replacing ethyl 2-bromopropionate with ethyl 4-bromobutyrate to obtain compound 3 with a yield of 78.5%. m.p.159~161℃.IRv max (cm -1 , KBr): 1732, 1666, 1612, 1381, 767. 1 H-NMR (400MHz, CDCl 3 ,δppm,J / Hz):1.28(t,J=7.2,3H),2.14~2.17(m,2H),2.53(t,J=7.2,2H),4.09(t,J=6,2H), 4.17(q, J=7.2, 2H), 6.36(d, J=2.0, 1H), 6.49(d, J=2.4, 1H), 6.67(s, 1H), 7.51~7.56(m, 3H), 7.88 (dd,J=1.2,7.6,2H),12.70(s,1H); 13 C-NMR (100MHz, DMSO-d6), δ: 14.08 (C-8"), 23.92 (C-3"), 29.97 (C-4"), 59.88 (C-7"), 67.44 (C-2 ”),92.80(C-8),98.56(C-6),105.12(C-3),105.42(C-10),126.38(C-2',C-6'),129.11(C-3' ,C-5'),130.63(C-4'),132.06(C-1'),157.46(C-9),161.58(C-2),163.18(C-5),164.42(C-7) ,172.39(C-5"),181.87(C-4); ES I-MS, m / z:369.1544[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com