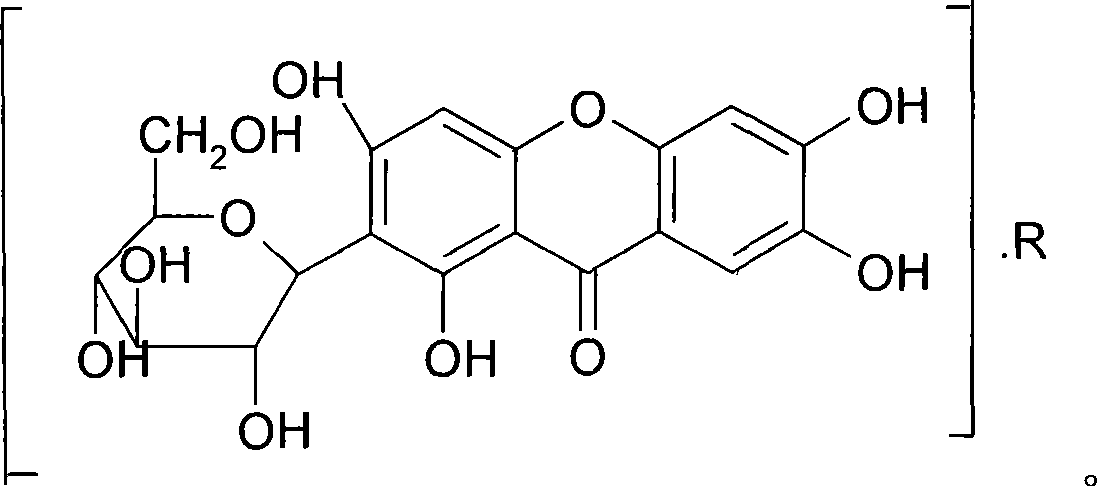
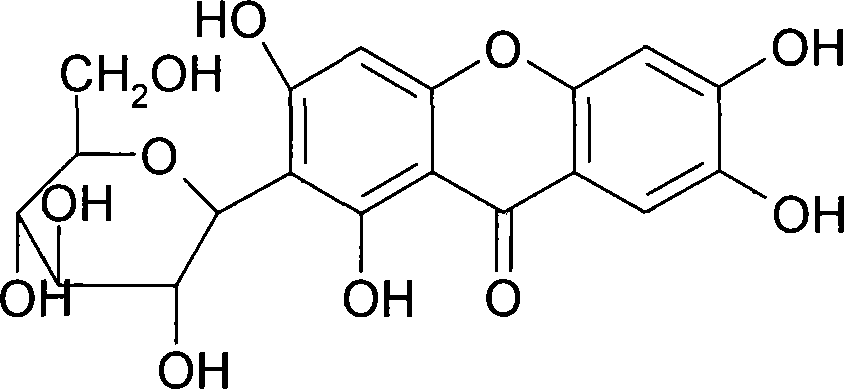
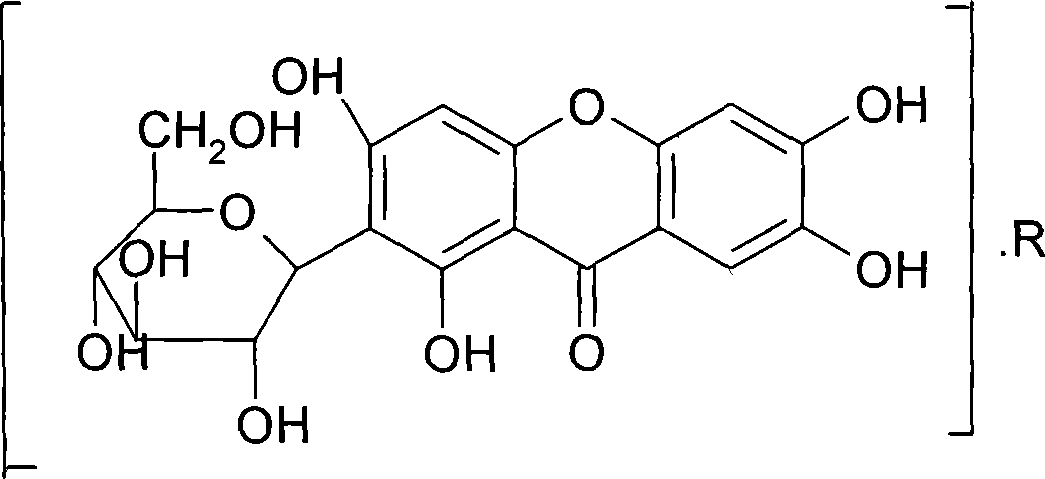
Mangiferin salt and method of preparing the same and use thereof
A technology of mangiferin and glycoside salts, which is applied in the preparation of sugar derivatives, chemical instruments and methods, and pharmaceutical formulations, etc., can solve the problems of low bioavailability, no drugs on the market, and no organic or inorganic salts of mangiferin.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0029] Preparation Example 1: Preparation of Mangiferin
[0030] Take 100 kg of Anemarrhena decoction pieces and put them in a multi-functional extraction tank, add 800 liters of 80% ethanol, reflux extraction for 1.5 hours, filter out the medicinal liquid, then add 600 liters of 80% ethanol, reflux extraction for 1.5 hours, filter out the medicinal liquid, and combine the medicinal liquids , decompression will collect ethanol until there is no alcohol smell. Take an appropriate amount of medicinal solution, add pretreated D101 macroporous resin for adsorption, wash with deionized water until clear; then elute with 30% ethanol, collect the eluent, concentrate the eluent, and dry under reduced pressure to obtain the product. As detected by phase chromatography, the purity of mangiferin was 95.4%.
Embodiment 1
[0031] Embodiment 1: the preparation of mangiferin arginine salt
[0032]Weigh 42.2g (0.1mol) of mangiferin and add it to 80g dimethyl sulfoxide to dissolve it, weigh 17.4g (0.1mol) of L-arginine and add it to 20g absolute ethanol to dissolve it, and stir the arginine solution slowly Add it to the mangiferin solution, stir until the reaction is complete, add an appropriate amount of absolute ethanol to the reaction solution to produce a large amount of light yellow precipitate, filter, and dry the precipitate under reduced pressure to obtain 56.2 g of a yellow-green or yellow solid, with a yield of 94.3%.
[0033] Compound Identification:
[0034] MSm / z: 595[M-H] + , 421[MG-H] + , 387, 369, 351, 173 [Arg-H] + .
[0035] 13 CNMR (DMSO-d 6 )(δppm): 162.3(C-1), 107.8(C-2), 168.3(C-3), 93.8(C-4), 156.0(C-4a), 150.9(C-4b), 102.8(C -5), 154.2(C-6), 143.8(C-7), 108.2(C-8), 111.8(C-8a), 101.5(C-8b).179.2(C=O), 81.6(C- 1'), 73.0(C-2'), 70.8(C-3'), 70.5(C-4'), 78.1(C-5'), 61.6(C...
Embodiment 2
[0037] Embodiment 2: the preparation of mangiferin arginine salt
[0038] Weigh 42.2g (0.1mol) of mangiferin and add an appropriate amount of 60% ethanol to dissolve it, weigh 52.2g (0.3mol) of L-arginine and add it to an appropriate amount of 80% ethanol to dissolve, slowly add mangiferin to the arginine solution while stirring solution, stirred until the reaction was complete, and an appropriate amount of acetone was added to the reaction solution to produce a large amount of light yellow precipitate, filtered, and the precipitate was dried under reduced pressure to obtain 75.8 g of a yellow-green or yellow solid, with a yield of 80.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com