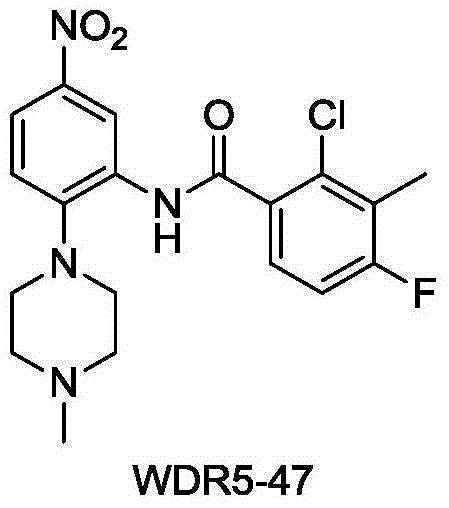
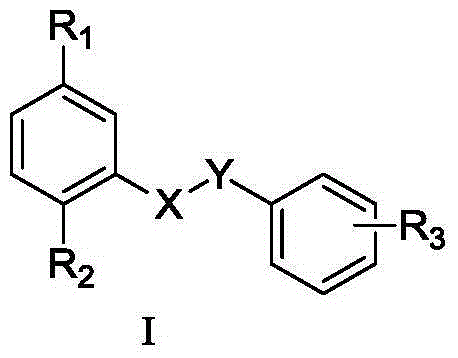
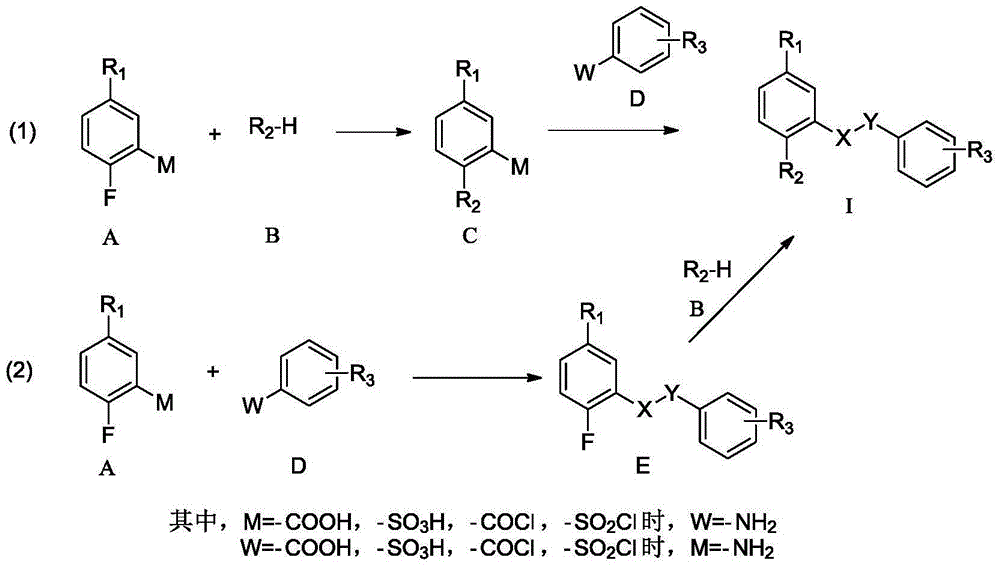
Amide compounds, preparation method and medical use thereof
A compound and pharmaceutical technology, applied in the preparation of organic compounds, carboxylic acid amide preparation, chemical instruments and methods, etc., can solve the problem of low activity of small molecule inhibitors, poor membrane permeability, poor physical and chemical properties of peptidomimetic compounds, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of N-(2-(4-methylpiperazine)-5-cyanophenyl)-2-chloro-3-methoxybenzenesulfonamide (1):
[0043] In a 25ml eggplant-shaped bottle, 1.0g (6.4mmol) of 2-fluoro-5-cyanoaniline was dissolved in anhydrous dichloromethane, and 2.3g (9.6mmol) of 2-chloro-3-methanol was added dropwise at room temperature. Dichloromethane solution of oxybenzenesulfonyl chloride, and then add 0.77ml (9.6mmol) of pyridine, and react overnight at room temperature. The reaction solution was spin-dried and column chromatographed to obtain 0.97 g of intermediate E as a light yellow solid (yield 48.5%).
[0044] Put 0.2g (0.61mmol) of the above solid in a 25ml eggplant-shaped bottle, dissolve it in 5ml DMF, add 0.088ml (0.8mmol) N-methylpiperazine, then add catalyst 0.14ml (0.8mmol) DIPEA, and react at 80°C for 3 After one hour, the reaction solution was diluted with 40 ml of ethyl acetate, washed twice with water, and the organic phase was collected and chromatographed to obtain 10.15 g of a ...
Embodiment 2
[0047] Synthesis of N-(2-fluoro-4-methoxyphenyl)-2-(4-methylhomopiperazine)-5-cyanophenylsulfonamide (2):
[0048] Using 2-fluoro-5-cyanobenzenesulfonyl chloride, 2-fluoro-4-methoxyaniline, and N-methylhomopiperazine as raw materials, according to the similar method of Example 1, compound 2 was synthesized, and two steps were obtained. rate 30.5%.
[0049] 1 HNMR (300MHz, CDCl 3 )δ8.22(s,1H),7.91(s,1H),7.81(d,J=7.3Hz,1H),7.08(d,J=8.4Hz,1H),6.98(d,J=7.7Hz, 2H),5.28(brs,1H),3.86(s,3H),3.28(t,J=4.3Hz,4H),3.02(m,2H),2.90(m,2H),2.70(s,3H), 1.87(m,2H).
Embodiment 3
[0051] Synthesis of N-(2-(4-methylpiperazine)-5-trifluoromethylphenyl)-2,4,6-trimethylbenzenesulfonamide (3):
[0052] Using 2-fluoro-5-trifluoromethylaniline, 2,4,6-trimethylbenzenesulfonyl chloride, and N-methylpiperazine as raw materials, compound 3 was synthesized according to the method similar to Example 1, two steps Yield 29.2%.
[0053] 1 HNMR (300MHz, CDCl 3 )δ7.98(d, J=2.5Hz, 1H), 7.86(dd, J=8.7, 2.5Hz, 2H), 7.23(d, J=8.7Hz, 1H), 6.98(s, 2H), 3.01( brs,4H),2.73(brs,10H),2.50(brs,4H),2.48(s,3H),2.28(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com