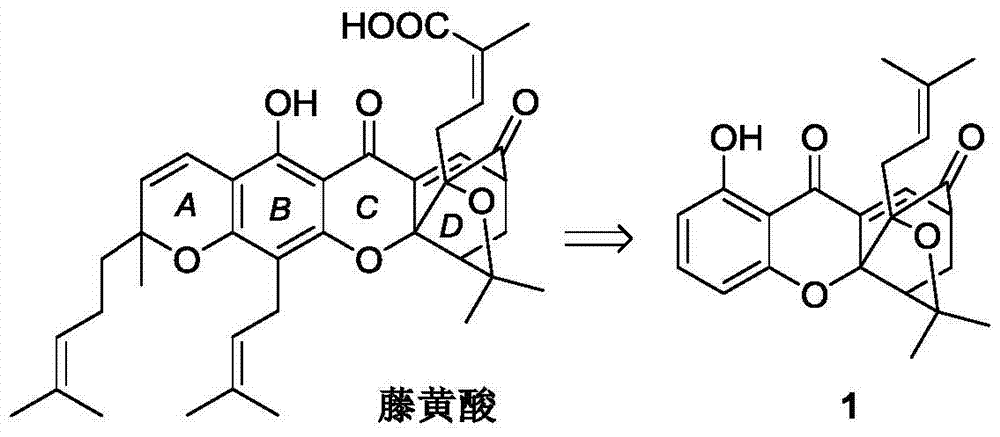
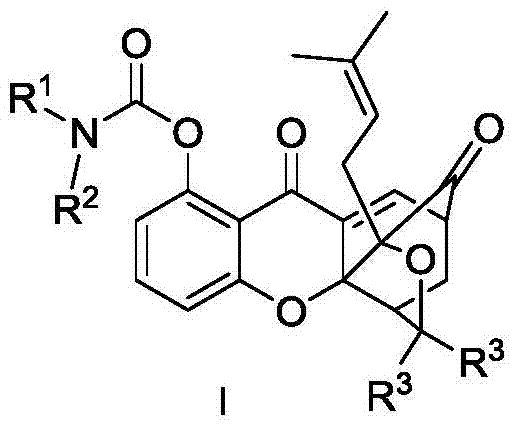
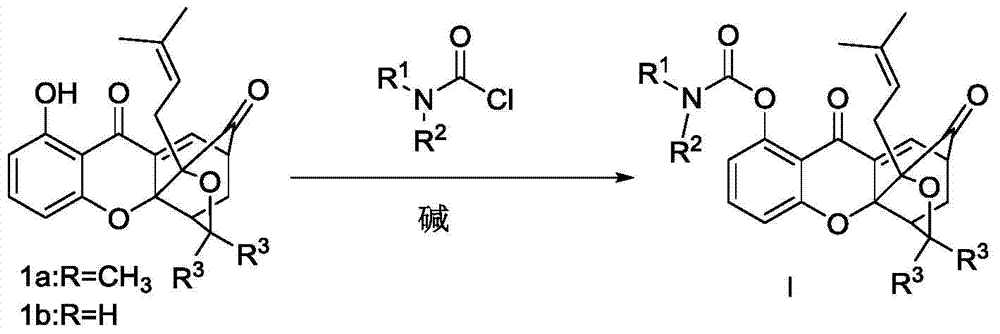
Carbamate bridged ring xanthone derivative, its preparation method and medical use
A pharmaceutical and alkyl technology, applied in the direction of anti-tumor drugs, drug combinations, organic chemistry, etc., can solve problems affecting anti-tumor activity and achieve good anti-tumor activity and novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 8-N,N-Dimethylcarbamoyloxy-3,3-dimethyl-1-(3-methylbut-2-en-1-yl)-3,3a,4,5-tetrahydro Synthesis of -1,5-methyl-1H,7H-furo[3,4-d]xanthene-7,13-dione (I-1)
[0043]
[0044] Compound 1a (80mg, 0.2mmol) was dissolved in acetone, and N,N-dimethylcarbamoyl chloride (34.24mg, 0.32mmol), potassium carbonate (43mg, 0.32mmol), DMAP (28mg, 0.24mmol) were added sequentially, N 2 protected and reacted overnight at room temperature.
[0045] The solvent was distilled off from the reaction solution under reduced pressure, and CH 2 Cl 2 dissolved, washed once with saturated ammonium chloride aqueous solution (20ml), washed with water (20ml), washed with saturated brine (20mL), anhydrous NaSO 4 Dry, filter with suction, and concentrate the filtrate to obtain a crude solid product, which is separated and purified by silica gel column chromatography (eluted with petroleum ether / ethyl acetate system) to obtain light yellow target product I-175 mg, with a yield of 76.53%. m.p.133.2...
Embodiment 2
[0047] 8-N,N-Diethylcarbamoyloxy-3,3-dimethyl-1-(3-methylbut-2-en-1-yl)-3,3a,4,5-tetrahydro Synthesis of -1,5-methyl-1H,7H-furo[3,4-d]xanthene-7,13-dione (I-2)
[0048]
[0049] Compound 1a (80mg, 0.2mmol) was dissolved in acetone, and N,N-diethylcarbamoyl chloride (43.24mg, 0.32mmol), triethylamine (0.044mL, 0.32mmol), DMAP (28mg, 0.24mmol) were added successively ), N 2 protected and reacted overnight at room temperature. The solvent was distilled off from the reaction solution under reduced pressure, and CH 2 Cl 2 dissolved, washed once with saturated ammonium chloride aqueous solution (20ml), washed with water (20ml), washed with saturated brine (20mL), anhydrous NaSO 4 Dry, filter with suction, and concentrate the filtrate to obtain a crude solid product, which is separated and purified by silica gel column chromatography (eluted with petroleum ether / ethyl acetate system) to obtain the light yellow target product I-279 mg, with a yield of 75.89%. m.p.144.6-146.4°C...
Embodiment 3
[0051] 8-N-methyl-N-ethylformyloxy-3,3-dimethyl-1-(3-methylbut-2-en-1-yl)-3,3a,4,5- Synthesis of Tetrahydro-1,5-methyl-1H,7H-furo[3,4-d]xanthene-7,13-dione (I-3)
[0052]
[0053]Compound 1a (80mg, 0.2mmol) was dissolved in dichloromethane, and N-methyl-Nethylcarbamoyl chloride (29.76mg, 0.32mmol), potassium carbonate (43mg, 0.32mmol), DMAP (28mg, 0.24mmol), N 2 protected and reacted overnight at room temperature. The solvent was distilled off from the reaction solution under reduced pressure, and CH 2 Cl 2 dissolved, washed once with saturated ammonium chloride aqueous solution (20ml), washed with water (20ml), washed with saturated brine (20mL), anhydrous NaSO 4 Dry, filter with suction, and concentrate the filtrate to obtain a crude solid, which is separated and purified by silica gel column chromatography (eluted with petroleum ether / ethyl acetate system) to obtain 368 mg of the white target product I-368 mg, with a yield of 73.11%. m.p.138-142°C; 1 H NMR (300MHz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com