New DHA derivatives and their use as medicaments
A technology of drugs and compounds, applied to compounds of general formula: field
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
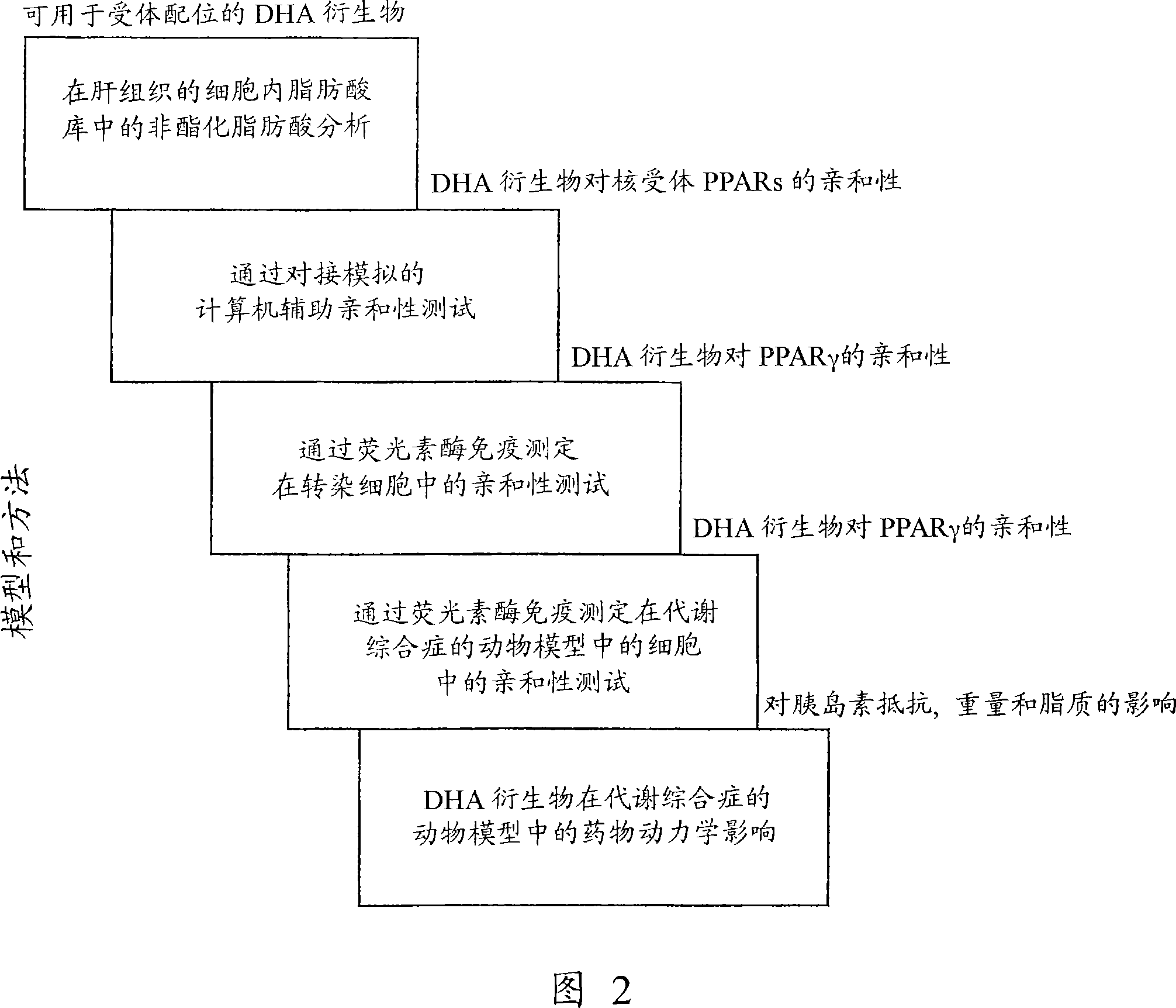
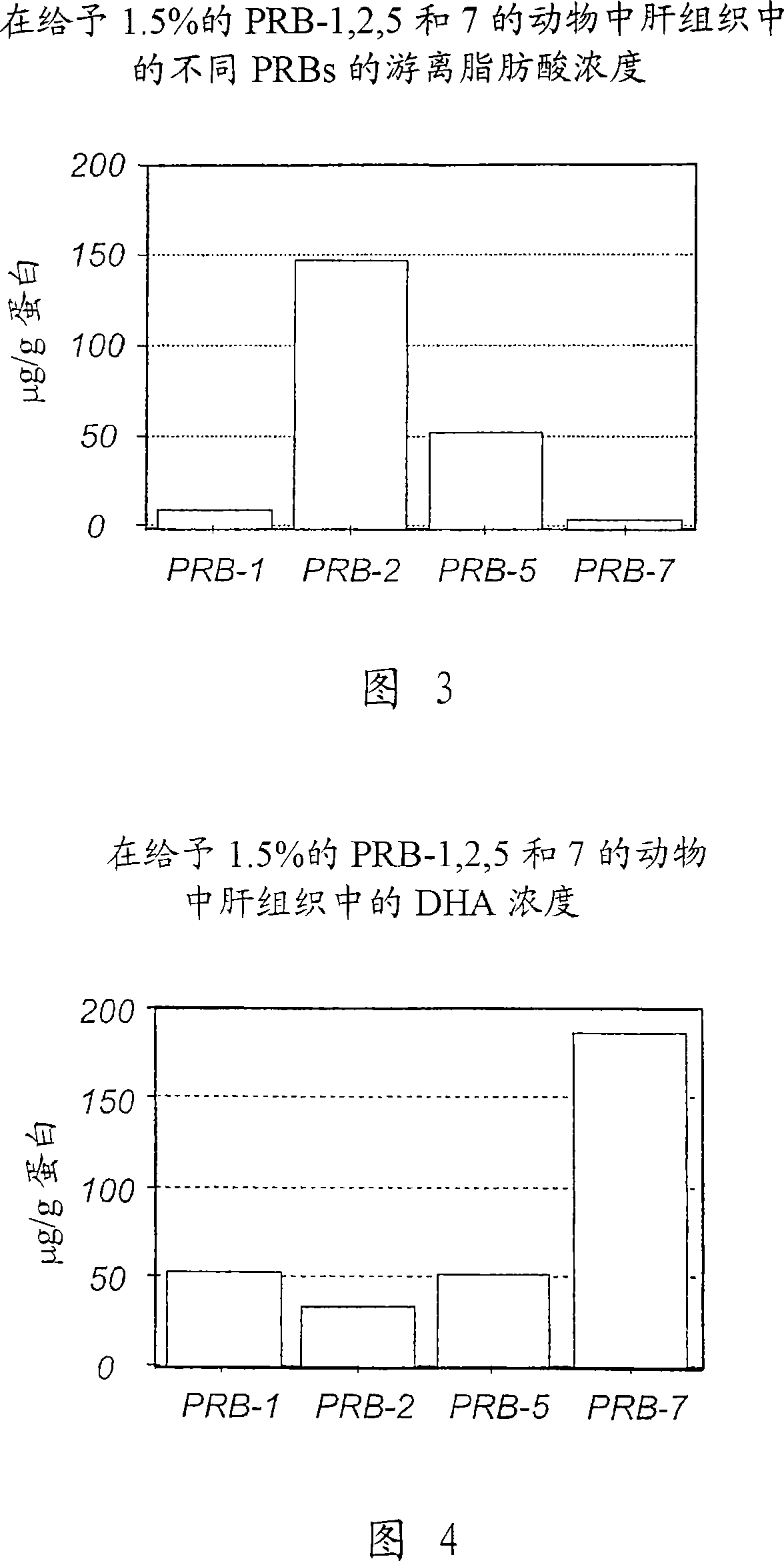
[0393] Example 1: Analysis of Intracellular Free Fatty Acids (Non-Esterified Fatty Acids) in Hepatocytes (Group 1 in Figure 2)
[0394] background
[0395] In the first set of experiments (Figure 2), free unesterified fatty acids were analyzed in liver tissue from animals dosed with PRB-1, 2, 5 and 7. These animals were transferred from the fifth set of experiments (Pharmacokinetic Effects of DHA Derivatives in Animal Models of Metabolic Syndrome). These animals have been given DHA (diet with 15% fat content) or DHA-derivatives (1.5% fat content in their diet) for 8 weeks and are assumed to be in a stable state with intracellular stable levels of DHA and DHA-derivatives. state. Since the rate of metabolism in the liver is very fast, liver tissue was selected.
[0396] method
[0397] The liver samples were homogenized in cold PBS buffer, immediately extracted with chloroform:methanol (2:1) containing 0.2 mM butylated hydroxytoluene (BHT), using cis-10- Heptadecenoic acid ...
Embodiment 2
[0404] Example 2: Computer Aided Affinity Test (Group 2 in Figure 2)
[0405] background
[0406] Nuclear receptors have been sequenced and amino acid sequences are known for PPARs and other related receptors used in the genetic control of glucose and fat. X-ray crystal structures and NMR spectra of PPARs are available, so computational affinity tests of fatty acid binding to receptors can be used to assess binding kinetics. Binding geometry, often referred to as binding mode or pose, includes both the position of the ligand relative to the receptor and the conformational state of the ligand and receptor, thus enabling the analysis of efficient ligand docking.
[0407] The affinity of the ligand for the receptor is defined by two distinct parameters: the docking of the ligand (DHA derivative) to the binding site of the receptor and the specific amino acid of the receptor and the carbonyl group or side chain in the fatty acid front. Electrostatic bonding (Krumrine).
[0408]...
Embodiment 3
[0423] Example 3: Affinity testing in transfected cells (Group 3 in Figure 2)
[0424] background
[0425] The release of luciferase correlates with the transcription of the gene. Binding of ligands to nuclear receptors such as PPARγ induces the transcription of the respective genes, thus releasing luciferase. This technique thus provides a means to measure ligand affinity for receptors and the activation of responsive genes.
[0426] method
[0427] Transient transfection of COS-1 cells in 6-well plates was performed as described by Graham and van der Eb (Graham). For full-length PPAR transfection studies, each well received 5 μg of the reporter construct, 2.5 μg of pSV-β-galactosidase as an internal control, 0.4 μg of pSG5-PPARγ2. Cells were harvested after 72 hours and luciferase activity was measured according to the protocol (Promega). Luciferase activity was normalized to β-galactosidase activity. Using 16 μl of lipofectamine Plus reagent, 4 μl of lipofectamine (Li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com