Low-dose doxepin formulations and methods of making and using the same
a technology of doxepin and low dose, which is applied in the direction of biocide, drug composition, organic non-active ingredients, etc., can solve the problems of complex and expensive equipment, complex granulation process, and difficulty in achieving acceptable content uniformity, and achieves the effect of minimizing fluidization, minimizing fluidization, and high content uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1 mg, 3 mg, and 6 mg Formulations
[0147]Examples of 1 mg, 3 mg, and 6 mg formulations are provided in Table 1 and Table 2.
TABLE 1Non-film coated tablets1 mg3 mg6 mgItemMaterial%Mg / tab%Mg / tab%Mg / tab1Doxepin HCl0.7531.132.263.394.526.782Silicified98.53147.8096.71145.0794.00141.00MicrocrystallineCellulose3Colloidal Silicon0.160.240.470.710.881.32Dioxide4FD&C Blue 1 Al Lake——0.050.080.020.0310-13%5DC Yellow 10 Al0.040.06——0.080.12Lake 36-42%6FD&C Yellow #6 Al0.010.015————Lake 15-18%7Magnesium Stearate0.500.750.500.750.500.75Totals:100.00150.00100.00150.00100.00150.00
TABLE 2Film-coated tablets1 mg3 mg6 mgItemMaterial%Mg / tab%Mg / tab%Mg / tab1Doxepin HCl0.7241.132.173.394.356.782Silicified94.79147.8893.04145.1590.48141.15MicrocrystallineCellulose3Colloidal Silicon0.150.240.460.710.851.32Dioxide4Magnesium Stearate0.480.750.480.750.480.755Film coat3.863.863.86Totals:100.00156.00100.00156.00100.00156.00
example 2
Doxepin Multimedia Dissolution Study
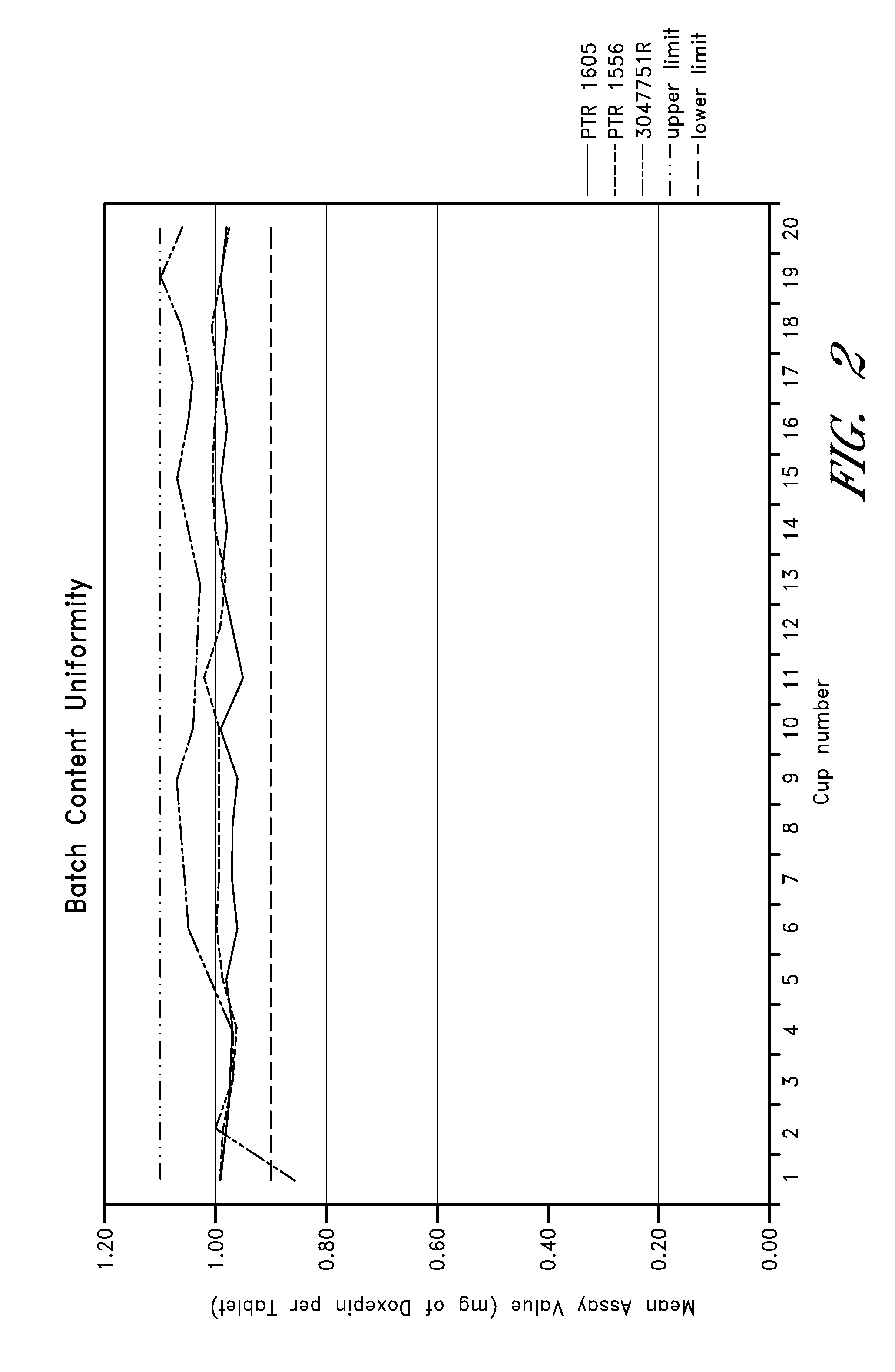
[0148]The dissolution of 1 mg (Lot Number 3047751R) and 6 mg (Lot Number 3047758R) SMCC-formulated, doxepin tablets in Simulated Gastric Fluid without enzymes (pH 1.2), 0.05 M acetate buffer (pH 4.5) and Simulated Intestinal Fluid USP without enzymes (pH 6.8) was measured with USP Apparatus 2 at 50 rpm using 900 mL of 37° C. i 0.5° C. dissolution media at 3, 5, 10, 15 and 30 minute time points. The average (n=12 tablets) percent doxepin released for each dosage strength in the two media at each time point is reported in Table 3.
TABLE 3Simulated Gastric0.05 M AcetateSimulated IntestinalFluid (pH 1.2)Buffer (pH 4.5)Fluid (pH 6.8)Time point1 mg6 mg1 mg6 mg1 mg6 mg3minutes*83% 70%*84% 71%55% 57%5minutes*91%*85%*93%*80%69% 72%10minutes*94%*90%*99%*91%79%*81%15minutes*96%*94%*101% *95%*81% *84%30minutes*97%*97%*102% *98%*86% *87%
[0149]The conditions with an asterisk in Table 2 achieve a Q value of 80% with none of the individual dissolution values falli...
example 4
Blend Uniformity
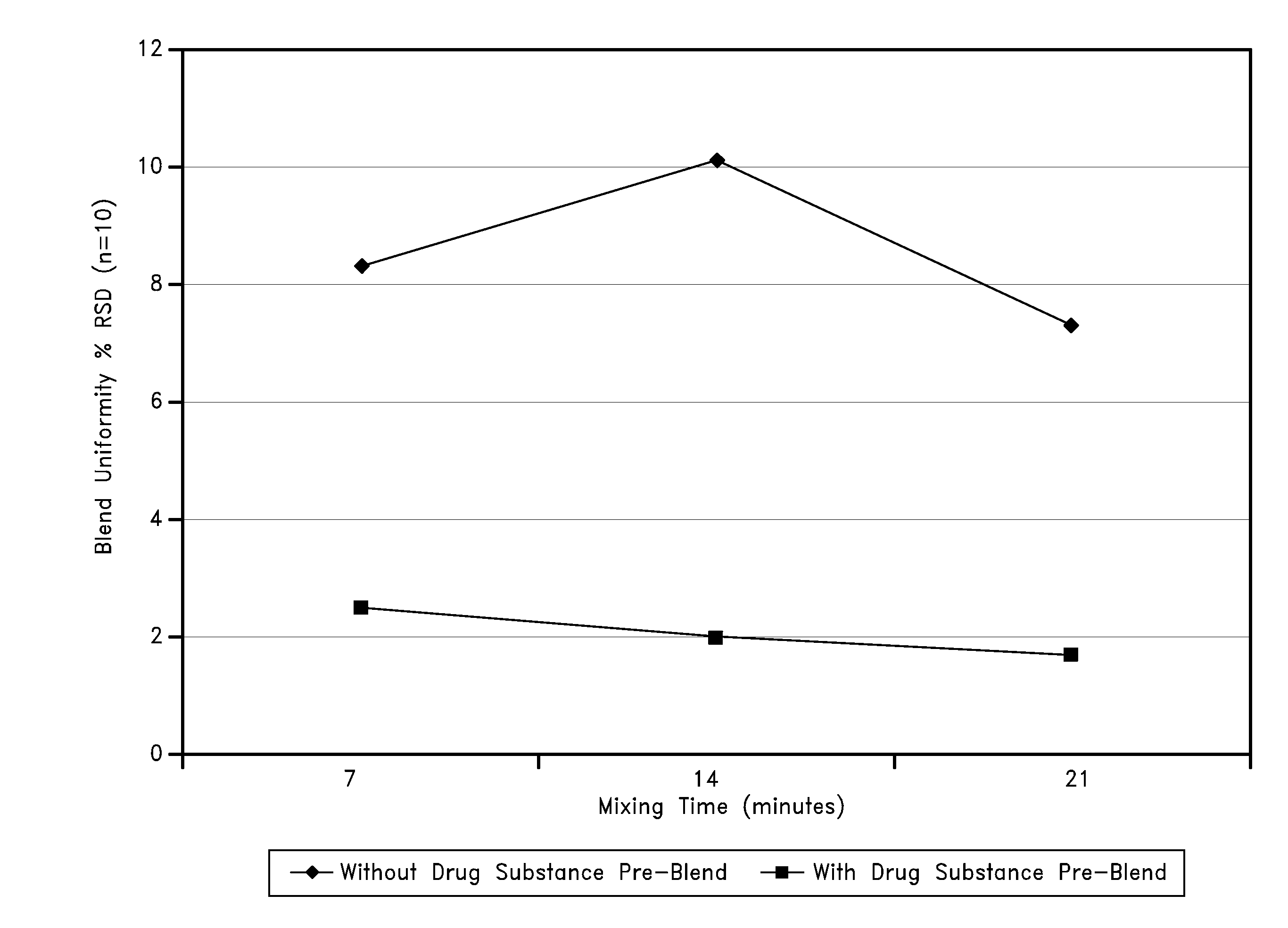
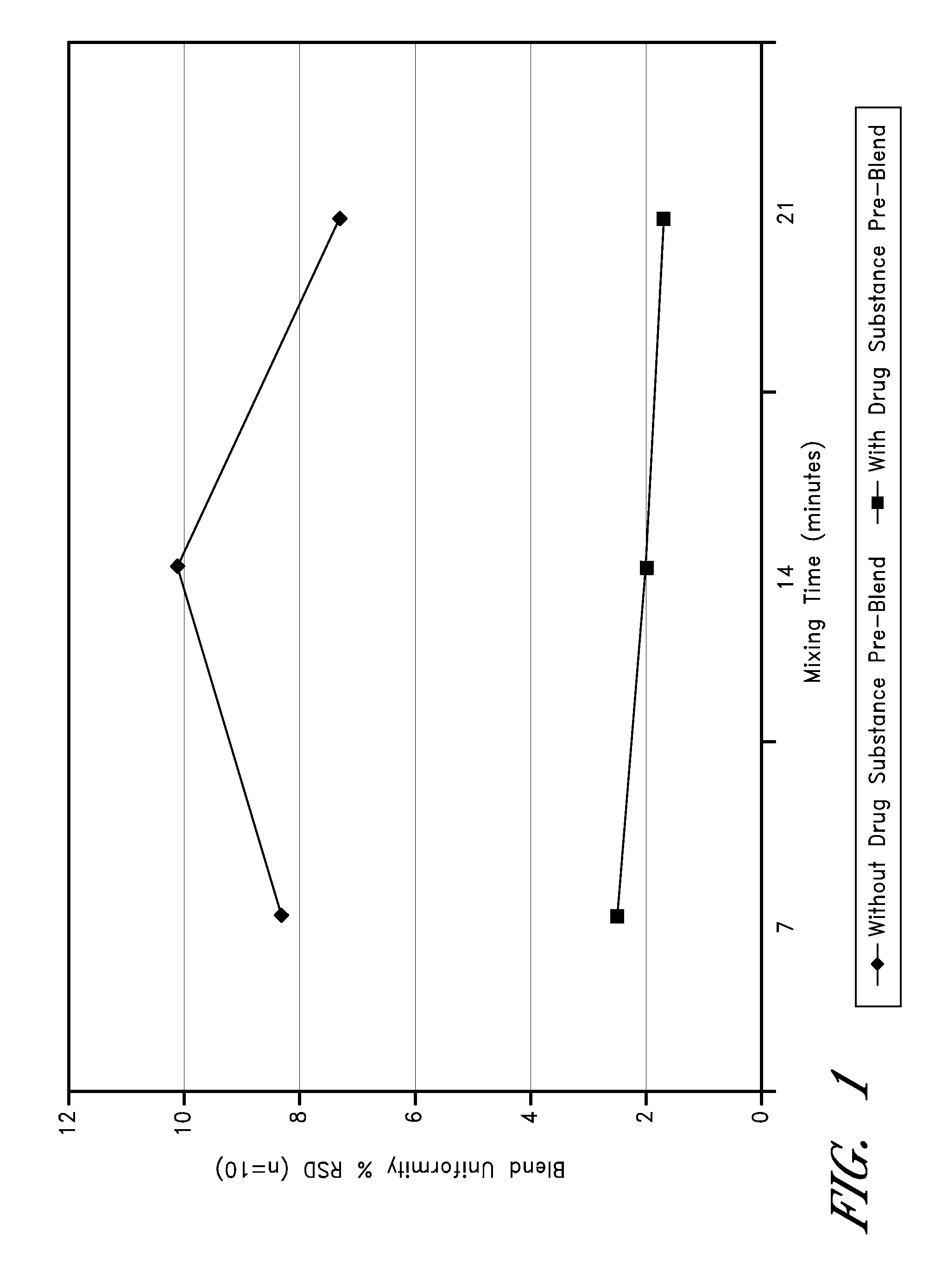
[0152]Due to the very low concentrations of drug substance in these tablet formulations, the blending process included preparation of a drug substance pre-blend created by layering doxepin HCl between additions of SMCC, followed by mixing. The uniformity of unit dose potency was further promoted by serially diluting and mixing the drug substance pre-blend with the remaining SMCC and colloidal silicon dioxide. FIG. 1 graphically illustrates the preparation of a drug substance pre-blend, which can result in the uniform distribution of drug substance in the drug product.
[0153]Thus, some embodiments relate to methods of improving blend uniformity, for example, by layering low dose doxepin with a filler, such as SMC C. It should be noted that other fillers can be used rather than SMCC or in addition to SMCC. Furthermore, uniformity can be improved by serially diluting the mixtures as described above with SMCC or any other filler or combination of fillers.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com